Identification and characterization of a sleep-active cell group in the rostral medullary brainstem
- PMID: 23238713
- PMCID: PMC3564016
- DOI: 10.1523/JNEUROSCI.0620-12.2012
Identification and characterization of a sleep-active cell group in the rostral medullary brainstem
Abstract
Early transection and stimulation studies suggested the existence of sleep-promoting circuitry in the medullary brainstem, yet the location and identity of the neurons comprising this putative hypnogenic circuitry remains unresolved. In the present study, we sought to uncover the location and identity of medullary neurons that might contribute to the regulation of sleep. Here we show the following in rats: (1) a delimited node of medullary neurons located lateral and dorsal to the facial nerve-a region we termed the parafacial zone (PZ)-project to the wake-promoting medial parabrachial nucleus; (2) PZ neurons express c-Fos after sleep but not after wakefulness and hence are sleep active; and (3) cell-body-specific lesions of the PZ result in large and sustained increases (50%) in daily wakefulness at the expense of slow-wave sleep (SWS). Using transgenic reporter mice [vesicular GABA/glycine transporter (Vgat)-GFP], we then show that >50% of PZ sleep-active neurons are inhibitory (GABAergic/glycinergic, VGAT-positive) in nature. Finally, we used a Cre-expressing adeno-associated viral vector and conditional Vgat(lox/lox) mice to selectively and genetically disrupt GABA/glycinergic neurotransmission from PZ neurons. Disruption of PZ GABAergic/glycinergic neurotransmission resulted in sustained increases (40%) in daily wakefulness at the expense of both SWS and rapid eye movement sleep. These results together reveal the location and neurochemical identity of a delimited node of sleep-active neurons within the rostral medullary brainstem.
Figures

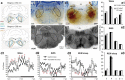
 , CTB and c-Fos double-labeled neurons; ▶, CTB single-labeled neurons; a2, a4), whereas only a few single-labeled c-Fos neurons and no double-labeled CTB and c-Fos neurons are seen in the awake rats (a3). c-Fos immunoreactivity is similarly seen in the PZ after sleeping (n = 3; b2) but not wake (n = 3; b1) episodes. Dual immunolabeling of c-Fos and GFP (as a proxy for VGAT-positive neurons) in the PZ after sleep (
, CTB and c-Fos double-labeled neurons; ▶, CTB single-labeled neurons; a2, a4), whereas only a few single-labeled c-Fos neurons and no double-labeled CTB and c-Fos neurons are seen in the awake rats (a3). c-Fos immunoreactivity is similarly seen in the PZ after sleeping (n = 3; b2) but not wake (n = 3; b1) episodes. Dual immunolabeling of c-Fos and GFP (as a proxy for VGAT-positive neurons) in the PZ after sleep ( , GFP and c-Fos double-labeled neurons; ▶, GFP single-labeled neurons; n = 2; c2) and wake (n = 2; c1) episodes in VGAT–GFP mice. Note that ∼45% of GFP-immunoreactive (VGAT+) neurons express c-Fos in the PZ after sleep, but no GFP/c-Fos double-labeled neurons are present in awake mice. 7n, facial nerve.
, GFP and c-Fos double-labeled neurons; ▶, GFP single-labeled neurons; n = 2; c2) and wake (n = 2; c1) episodes in VGAT–GFP mice. Note that ∼45% of GFP-immunoreactive (VGAT+) neurons express c-Fos in the PZ after sleep, but no GFP/c-Fos double-labeled neurons are present in awake mice. 7n, facial nerve.
References
-
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, Akaoka H, Sergeeva OA, Yanagisawa M, Ohtsu H, Franco P, Haas HL, Lin JS. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–14438. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
