Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling
- PMID: 23238714
- PMCID: PMC6621721
- DOI: 10.1523/JNEUROSCI.3069-12.2012
Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling
Abstract
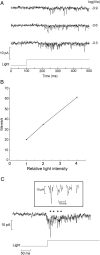
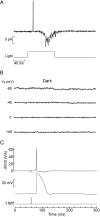
Melanopsin, a photopigment related to the rhodopsin of microvillar photoreceptors of invertebrates, evolved in vertebrates to subserve nonvisual light-sensing functions, such as the pupillary reflex and entrainment of circadian rhythms. However, vertebrate circadian receptors display no hint of a microvillar specialization and show an extremely low light sensitivity and sluggish kinetics. Recently in amphioxus, the most basal chordate, melanopsin-expressing photoreceptors were characterized; these cells share salient properties with both rhabdomeric photoreceptors of invertebrates and circadian receptors of vertebrates. We used electrophysiology to dissect the gain of the light-transduction process in amphioxus and examine key features that help outline the evolutionary transition toward a sensor optimized to report mean ambient illumination rather than mediating spatial vision. By comparing the size of current fluctuations attributable to single photon melanopsin isomerizations with the size of single-channels activated by light, we concluded that the gain of the transduction cascade is lower than in rhabdomeric receptors. In contrast, the expression level of melanopsin (gauged by measuring charge displacements during photo-induced melanopsin isomerization) is comparable with that of canonical visual receptors. A modest amplification in melanopsin-using receptors is therefore apparent in early chordates; the decrease in photopigment expression-and loss of the anatomical correlates-observed in vertebrates subsequently enabled them to attain the low photosensitivity tailored to the role of circadian receptors.
Figures





References
-
- Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563–571. - PubMed
-
- Arendt D, Tessmar K, de Campos-Baptista MI, Dorresteijn A, Wittbrodt J. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development. 2002;129:1143–1154. - PubMed
-
- Bacigalupo J, Lisman JE. Single channel currents activated by light in Limulus ventral photoreceptors. Nature. 1983;304:268–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
