Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects
- PMID: 23238854
- PMCID: PMC3610676
- DOI: 10.1210/er.2012-1008
Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects
Abstract
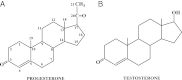
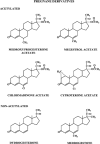
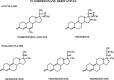
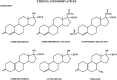
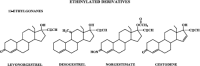
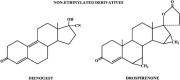
The safety of progestogens as a class has come under increased scrutiny after the publication of data from the Women's Health Initiative trial, particularly with respect to breast cancer and cardiovascular disease risk, despite the fact that only one progestogen, medroxyprogesterone acetate, was used in this study. Inconsistency in nomenclature has also caused confusion between synthetic progestogens, defined here by the term progestin, and natural progesterone. Although all progestogens by definition have progestational activity, they also have a divergent range of other properties that can translate to very different clinical effects. Endometrial protection is the primary reason for prescribing a progestogen concomitantly with postmenopausal estrogen therapy in women with a uterus, but several progestogens are known to have a range of other potentially beneficial effects, for example on the nervous and cardiovascular systems. Because women remain suspicious of the progestogen component of postmenopausal hormone therapy in the light of the Women's Health Initiative trial, practitioners should not ignore the potential benefits to their patients of some progestogens by considering them to be a single pharmacological class. There is a lack of understanding of the differences between progestins and progesterone and between individual progestins differing in their effects on the cardiovascular and nervous systems, the breast, and bone. This review elucidates the differences between the substantial number of individual progestogens employed in postmenopausal hormone therapy, including both progestins and progesterone. We conclude that these differences in chemical structure, metabolism, pharmacokinetics, affinity, potency, and efficacy via steroid receptors, intracellular action, and biological and clinical effects confirm the absence of a class effect of progestogens.
Figures
References
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 - PubMed
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, et al. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701–1712 - PubMed
-
- Stanczyk FZ, Henzl MR. 2001. Use of the name “Progestin.” Contraception 64:1–2 - PubMed
-
- North American Menopause Society 2003. Role of progestogen in hormone therapy for postmenopausal women: position statement of the North American Menopause Society. Menopause 10:113–132 - PubMed
-
- Stanczyk FZ. 2003. All progestins are not created equal. Steroids 68:879–890 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

