The endocannabinoid N-arachidonoyl glycine (NAGly) inhibits store-operated Ca2+ entry by preventing STIM1-Orai1 interaction
- PMID: 23239024
- PMCID: PMC3614444
- DOI: 10.1242/jcs.118075
The endocannabinoid N-arachidonoyl glycine (NAGly) inhibits store-operated Ca2+ entry by preventing STIM1-Orai1 interaction
Abstract
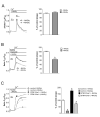
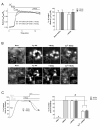
The endocannabiniod anandamide (AEA) and its derivate N-arachidonoyl glycine (NAGly) have a broad spectrum of physiological effects, which are induced by both binding to receptors and receptor-independent modulations of ion channels and transporters. The impact of AEA and NAGly on store-operated Ca(2+) entry (SOCE), a ubiquitous Ca(2+) entry pathway regulating many cellular functions, is unknown. Here we show that NAGly, but not AEA reversibly hinders SOCE in a time- and concentration-dependent manner. The inhibitory effect of NAGly on SOCE was found in the human endothelial cell line EA.hy926, the rat pancreatic β-cell line INS-1 832/13, and the rat basophilic leukemia cell line RBL-2H3. NAGly diminished SOCE independently from the mode of Ca(2+) depletion of the endoplasmic reticulum, whereas it had no effect on Ca(2+) entry through L-type voltage-gated Ca(2+) channels. Enhanced Ca(2+) entry was effectively hampered by NAGly in cells overexpressing the key molecular constituents of SOCE, stromal interacting molecule 1 (STIM1) and the pore-forming subunit of SOCE channels, Orai1. Fluorescence microscopy revealed that NAGly did not affect STIM1 oligomerization, STIM1 clustering, or the colocalization of STIM1 with Orai1, which were induced by Ca(2+) depletion of the endoplasmic reticulum. In contrast, independently from its slow depolarizing effect on mitochondria, NAGly instantly and strongly diminished the interaction of STIM1 with Orai1, indicating that NAGly inhibits SOCE primarily by uncoupling STIM1 from Orai1. In summary, our findings revealed the STIM1-Orai1-mediated SOCE machinery as a molecular target of NAGly, which might have many implications in cell physiology.
Figures


References
-
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J Biol Chem. 2012;287:34445–34454. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

