CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells
- PMID: 23239029
- PMCID: PMC4074255
- DOI: 10.1242/jcs.119826
CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells
Abstract
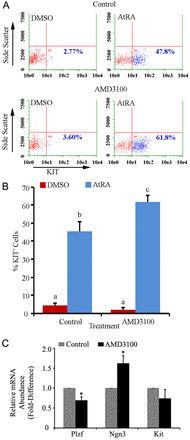
Continual spermatogenesis relies on the activities of a tissue-specific stem cell population referred to as spermatogonial stem cells (SSCs). Fate decisions of stem cells are influenced by their niche environments, a major component of which is soluble factors secreted by support cells. At present, the factors that constitute the SSC niche are undefined. We explored the role of chemokine (C-X-C motif) ligand 12 (CXCL12) signaling via its receptor C-X-C chemokine receptor type 4 (CXCR4) in regulation of mouse SSC fate decisions. Immunofluorescent staining for CXCL12 protein in cross sections of testes from both pup and adult mice revealed its localization at the basement membrane of seminiferous tubules. Within the undifferentiated spermatogonial population of mouse testes, a fraction of cells were found to express CXCR4 and possess stem cell capacity. Inhibition of CXCR4 signaling in primary cultures of mouse undifferentiated spermatogonia resulted in SSC loss, in part by reducing proliferation and increasing the transition to a progenitor state primed for differentiation upon stimulation by retinoic acid. In addition, CXCL12-CXCR4 signaling in mouse SSCs was found to be important for colonization of recipient testes following transplantation, possibly by influencing homing to establish stem-cell niches. Furthermore, inhibition of CXCR4 signaling in testes of adult mice impaired SSC maintenance, leading to loss of the germline. Collectively, these findings indicate that CXCL12 is an important component of the growth factor milieu of stem cells in mammalian testes and that it signals via the CXCR4 to regulate maintenance of the SSC pool.
Figures





References
-
- Ara T., Nakamura Y., Egawa T., Sugiyama T., Abe K., Kishimoto T., Matsui Y., Nagasawa T. (2003). Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc. Natl. Acad. Sci. USA 100, 5319–5323 10.1073/pnas.0730719100 - DOI - PMC - PubMed
-
- Arai A., Jin A., Yan W., Mizuchi D., Yamamoto K., Nanki T., Miura O. (2005). SDF-1 synergistically enhances IL-3-induced activation of the Raf-1/MEK/Erk signaling pathway through activation of Rac and its effector Pak kinases to promote hematopoiesis and chemotaxis. Cell. Signal. 17, 497–506 10.1016/j.cellsig.2004.09.007 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

