Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge
- PMID: 23239495
- PMCID: PMC3604083
- DOI: 10.1165/rcmb.2012-0146OC
Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge
Abstract
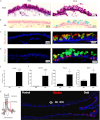
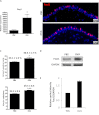
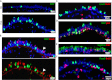
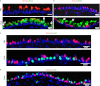
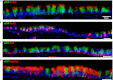
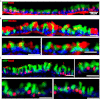
Mucous cell metaplasia is a hallmark of airway diseases, such as asthma and chronic obstructive pulmonary disease. The majority of human airway epithelium is pseudostratified, but the cell of origin of mucous cells has not been definitively established in this type of airway epithelium. There is evidence that ciliated, club cell (Clara), and basal cells can all give rise to mucus-producing cells in different contexts. Because pseudostratified airway epithelium contains distinct progenitor cells from simple columnar airway epithelium, the lineage relationships of progenitor cells to mucous cells may be different in these two epithelial types. We therefore performed lineage tracing of the ciliated cells of the murine basal cell-containing airway epithelium in conjunction with the ovalbumin (OVA)-induced murine model of allergic lung disease. We genetically labeled ciliated cells with enhanced Yellow Fluorescent Protein (eYFP) before the allergen challenge, and followed the fate of these cells to determine whether they gave rise to newly formed mucous cells. Although ciliated cells increased in number after the OVA challenge, the newly formed mucous cells were not labeled with the eYFP lineage tag. Even small numbers of labeled mucous cells could not be detected, implying that ciliated cells make virtually no contribution to the new goblet cell pool. This demonstrates that, after OVA challenge, new mucous cells do not originate from ciliated cells in a pseudostratified basal cell-containing airway epithelium.
Figures

References
-
- Aikawa T, Shimura S, Sasaki H, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921 - PubMed
-
- Vestbo J. Epidemiological studies in mucus hypersecretion. Novartis Found Symp 2002;248:3–19 - PubMed
-
- Rogers DF. The airway goblet cell. Int J Biochem Cell Biol 2003;35:1–6 - PubMed
-
- Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 2006;38:116–125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

