Proteomic analysis and immunogenicity of Mannheimia haemolytica vesicles
- PMID: 23239798
- PMCID: PMC3571273
- DOI: 10.1128/CVI.00622-12
Proteomic analysis and immunogenicity of Mannheimia haemolytica vesicles
Abstract
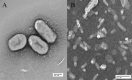
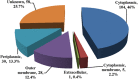
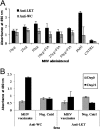
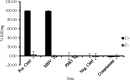
Mannheimia haemolytica, a major causative agent in bovine respiratory disease, inflicts extensive losses each year on cattle producers. Commercially available vaccines are only partially efficacious. Immunity to M. haemolytica requires antibodies to secreted toxins and outer membrane proteins (OMPs) of the bacterium. Gram-negative bacteria produce membrane blebs or vesicles, the membrane components of which are primarily derived from OMPs. Accordingly, vesicles have been used as immunogens with various degrees of success. This study characterized components of M. haemolytica vesicles and determined their immunogenicity in mice and cattle. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of vesicles from this bacterium identified 226 proteins, of which 58 (25.6%) were OMPs and periplasmic and one (0.44%) was extracellular. Vesicles were used to vaccinate dairy calves and BALB/c mice. Analyses of sera from calves and mice by enzyme-linked immunosorbent assay (ELISA) showed that circulating antibodies against M. haemolytica whole cells and leukotoxin were significantly higher on days 21 and 28 (P < 0.05) than on day 0. For control calves and mice, there were no significant differences in serum anti-whole-cell and leukotoxin antibody levels from days 0 and 21 or 28, respectively. Lesion scores of lungs from vaccinated calves (15.95%) were significantly (P < 0.05) lower than those from nonvaccinated calves (42.65%). Sera from mice on day 28 and calves on day 21 showed 100% serum bactericidal activity. Sera from vesicle-vaccinated mice neutralized leukotoxin.
Figures
References
-
- Perino LJ, Hunsaker BD. 1997. A review of bovine respiratory disease vaccine field efficacy. Bovine Practitioner 31:59–66
-
- Confer AW. 1993. Immunogens of Pasteurella. Vet. Microbiol. 37:353–368 - PubMed
-
- Ayalew S, Confer AW, Hartson SD, Shrestha B. 2010. Immunoproteomic analyses of outer membrane proteins of Mannheimia haemolytica and identification of potential vaccine candidates. Proteomics 10:2151–2164 - PubMed
-
- Ayalew S, Shrestha B, Montelongo M, Wilson A, Confer AW. 27 August 2011. Identification and immunogenicity of Mannheimia haemolytica S1 outer membrane lipoprotein PlpF. Vaccine [Epub ahead of print.] doi:10.1016/j.vaccine.2011.08.074 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

