Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies
- PMID: 23239801
- PMCID: PMC3571260
- DOI: 10.1128/CVI.00356-12
Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies
Abstract
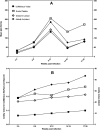
Toxoplasma infection in pregnant women may cause congenital toxoplasmosis. Diagnosis of infection is based on serological tests aimed at detecting IgM and IgG antibodies against Toxoplasma gondii. However, IgM antibodies are not an accurate marker for discriminating between acute and latent infection. Detection of residual or persistent IgM may occur months or even years after primary infection, while the IgG avidity test is a rapid means of identifying latent infections in pregnant women who exhibit both IgG and IgM anti-Toxoplasma antibodies on initial testing during pregnancy. In this study, we assessed and compared the performances of four commercially available Toxoplasma IgG avidity tests in immunocompetent and immunocompromised patients with acute and latent toxoplasmosis. The positive predictive value of high avidity to confirm latent toxoplasmosis was 100% for all the assays, indicating that high avidity is a hallmark of latent infection. However, the negative predictive value of high avidity ranged from 99.2% (bioMérieux) to 95.3% (Abbott), indicating that acute toxoplasmosis could not be reliably diagnosed based on low IgG avidity alone. Thus, the avidity test provides a rapid means for identifying latent Toxoplasma infection in immunocompetent pregnant women presenting both IgG and IgM anti-Toxoplasma antibodies on initial testing. In terms of cost-effectiveness, avidity testing is a powerful tool that optimizes screening and follow-up of pregnant women while minimizing the costs of screening by avoiding subsequent costly maternal and fetal investigation and unnecessary treatment. The cheapest assay, Vidas Toxo IgG Avidity, also had the best performance for the diagnosis of latent toxoplasmosis.
Figures



References
-
- Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554–566 - PubMed
-
- Couvreur J. 1962. Congenital toxoplasmosis. Vie Med. 43:1839–1842 - PubMed
-
- Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A, Villena I, Jenum PA, Hayde M, Naessens A. 1999. Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. Am. J. Obstet. Gynecol. 181:843–847 - PubMed
-
- Villard O, Jung-Etienne J, Cimon B, Franck J, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E, Le Reseau du Centre National de Référence de la Toxoplasmose. 2011. Sérodiagnostic de la toxoplasmose en 2010: conduite à tenir et interpretation en fonction des profils sérologiques obtenus par les méthodes de dépisage. Feuillets Biol. 52:1–7
-
- Pratlong F. 2002. Toxoplasmosis and pregnancy: current trends in serological follow-up. Gynecol. Obstet. Fertil. 30:237–243 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

