LEOPARD Syndrome: Clinical Features and Gene Mutations
- PMID: 23239957
- PMCID: PMC3507272
- DOI: 10.1159/000342251
LEOPARD Syndrome: Clinical Features and Gene Mutations
Abstract
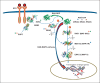
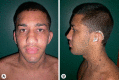
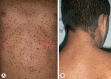
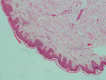
The RAS/MAPK pathway proteins with germline mutations in their respective genes are associated with some disorders such as Noonan, LEOPARD (LS), neurofibromatosis type 1, Costello and cardio-facio-cutaneous syndromes. LEOPARD is an acronym, mnemonic for the major manifestations of this disorder, characterized by multiple lentigines, electrocardiographic abnormalities, ocular hypertelorism, pulmonic stenosis, abnormal genitalia, retardation of growth, and sensorineural deafness. Though it is not included in the acronym, hypertrophic cardiomyopathy is the most frequent cardiac anomaly observed, representing a potentially life-threatening problem in these patients. PTPN11, RAF1 and BRAF are the genes known to be associated with LS, identifying molecular genetic testing of the 3 gene mutations in about 95% of affected individuals. PTPN11 mutations are the most frequently found. Eleven different missense PTPN11 mutations (Tyr279Cys/Ser, Ala461Thr, Gly464Ala, Thr468Met/Pro, Arg498Trp/Leu, Gln506Pro, and Gln510Glu/Pro) have been reported so far in LS, 2 of which (Tyr279Cys and Thr468Met) occur in about 65% of the cases. Here, we provide an overview of clinical aspects of this disorder, the molecular mechanisms underlying pathogenesis and major genotype-phenotype correlations.
Keywords: BRAF; Gene; LEOPARD; Mutation; PTPN11; RAF1; RAR/MAPK pathway.
Figures

References
-
- Adriaenssens T, Ibrahim T, Seyfarth M. The LEOPARD syndrome: a rare condition associated with hypertrophic cardiomyopathy. Eur Heart J. 2007;28:3066. - PubMed
-
- Alfieri P, Cesarini L, Zampino G, Pantaleoni F, Selicorni A, et al. Visual function in Noonan and LEOPARD syndrome. Neuropediatrics. 2008;39:335–340. - PubMed
-
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. - PubMed
-
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29:992–1006. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

