Baicalin downregulates Porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling
- PMID: 23239998
- PMCID: PMC3519831
- DOI: 10.1371/journal.pone.0051008
Baicalin downregulates Porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling
Abstract
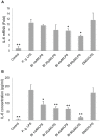
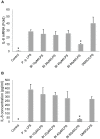
Periodontal (gum) disease is one of the main global oral health burdens and severe periodontal disease (periodontitis) is a leading cause of tooth loss in adults globally. It also increases the risk of cardiovascular disease and diabetes mellitus. Porphyromonas gingivalis lipopolysaccharide (LPS) is a key virulent attribute that significantly contributes to periodontal pathogenesis. Baicalin is a flavonoid from Scutellaria radix, an herb commonly used in traditional Chinese medicine for treating inflammatory diseases. The present study examined the modulatory effect of baicalin on P. gingivalis LPS-induced expression of IL-6 and IL-8 in human oral keratinocytes (HOKs). Cells were pre-treated with baicalin (0-80 µM) for 24 h, and subsequently treated with P. gingivalis LPS at 10 µg/ml with or without baicalin for 3 h. IL-6 and IL-8 transcripts and proteins were detected by real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The expression of nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) proteins was analyzed by western blot. A panel of genes related to toll-like receptor (TLR) signaling was examined by PCR array. We found that baicalin significantly downregulated P. gingivalis LPS-stimulated expression of IL-6 and IL-8, and inhibited P. gingivalis LPS-activated NF-κB, p38 MAPK and JNK. Furthermore, baicalin markedly downregulated P. gingivalis LPS-induced expression of genes associated with TLR signaling. In conclusion, the present study shows that baicalin may significantly downregulate P. gingivalis LPS-upregulated expression of IL-6 and IL-8 in HOKs via negative regulation of TLR signaling.
Conflict of interest statement
Figures


References
-
- Jin LJ, Armitage GC, Klinge B, Lang NP, Tonetti M, et al. (2011) Global oral health inequalities: Task group-periodontal disease. Adv Dent Res 23: 221–226. - PubMed
-
- Li X, Tse HF, Jin LJ (2011) Novel endothelial biomarkers: implications for periodontal disease and CVD. J Dent Res 90: 1062–1069. - PubMed
-
- Lalla E, Papapanou PN (2011) Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7: 738–748. - PubMed
-
- Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8: 481–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

