doublesex functions early and late in gustatory sense organ development
- PMID: 23240029
- PMCID: PMC3519885
- DOI: 10.1371/journal.pone.0051489
doublesex functions early and late in gustatory sense organ development
Abstract
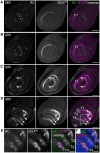
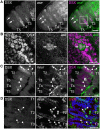
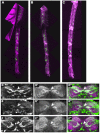
Somatic sexual dimorphisms outside of the nervous system in Drosophila melanogaster are largely controlled by the male- and female-specific Doublesex transcription factors (DSX(M) and DSX(F), respectively). The DSX proteins must act at the right times and places in development to regulate the diverse array of genes that sculpt male and female characteristics across a variety of tissues. To explore how cellular and developmental contexts integrate with doublesex (dsx) gene function, we focused on the sexually dimorphic number of gustatory sense organs (GSOs) in the foreleg. We show that DSX(M) and DSX(F) promote and repress GSO formation, respectively, and that their relative contribution to this dimorphism varies along the proximodistal axis of the foreleg. Our results suggest that the DSX proteins impact specification of the gustatory sensory organ precursors (SOPs). DSX(F) then acts later in the foreleg to regulate gustatory receptor neuron axon guidance. These results suggest that the foreleg provides a unique opportunity for examining the context-dependent functions of DSX.
Conflict of interest statement
Figures


References
-
- Gehring WJ (1996) The master control gene for morphogenesis and evolution of the eye. Genes Cells 1: 11–15. - PubMed
-
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, et al. (1996) Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382: 133–138. - PubMed
-
- Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801. - PubMed
-
- Cline TW, Meyer BJ (1996) Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30: 637–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

