Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve
- PMID: 23240031
- PMCID: PMC3519875
- DOI: 10.1371/journal.pone.0051499
Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve
Abstract
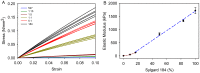
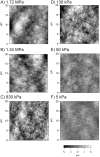
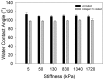
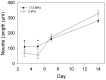
Mechanics is an important component in the regulation of cell shape, proliferation, migration and differentiation during normal homeostasis and disease states. Biomaterials that match the elastic modulus of soft tissues have been effective for studying this cell mechanobiology, but improvements are needed in order to investigate a wider range of physicochemical properties in a controlled manner. We hypothesized that polydimethylsiloxane (PDMS) blends could be used as the basis of a tunable system where the elastic modulus could be adjusted to match most types of soft tissue. To test this we formulated blends of two commercially available PDMS types, Sylgard 527 and Sylgard 184, which enabled us to fabricate substrates with an elastic modulus anywhere from 5 kPa up to 1.72 MPa. This is a three order-of-magnitude range of tunability, exceeding what is possible with other hydrogel and PDMS systems. Uniquely, the elastic modulus can be controlled independently of other materials properties including surface roughness, surface energy and the ability to functionalize the surface by protein adsorption and microcontact printing. For biological validation, PC12 (neuronal inducible-pheochromocytoma cell line) and C2C12 (muscle cell line) were used to demonstrate that these PDMS formulations support cell attachment and growth and that these substrates can be used to probe the mechanosensitivity of various cellular processes including neurite extension and muscle differentiation.
Conflict of interest statement
Figures




References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. - PubMed
-
- Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143. - PubMed
-
- Discher DE, Engler A, Carag C, Rehfeldt F (2008) Matrix elasticity effects on cardiomyocytes and stem cells: Similarities, differences and therapeutic implications. Biorheology 45: 54–54.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

