High yield production and refolding of the double-knot toxin, an activator of TRPV1 channels
- PMID: 23240036
- PMCID: PMC3519854
- DOI: 10.1371/journal.pone.0051516
High yield production and refolding of the double-knot toxin, an activator of TRPV1 channels
Abstract
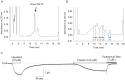
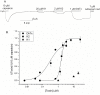
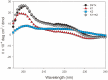
A unique peptide toxin, named double-knot toxin (DkTx), was recently purified from the venom of the tarantula Ornithoctonus huwena and was found to stably activate TRPV1 channels by targeting the outer pore domain. DkTx has been shown to consist of two inhibitory cysteine-knot (ICK) motifs, referred to as K1 and K2, each containing six cysteine residues. Beyond this initial characterization, however, the structural and functional details about DkTx remains elusive in large part due to the lack of a high yielding methodology for the synthesis and folding of this cysteine-rich peptide. Here, we overcome this obstacle by generating pure DkTx in quantities sufficient for structural and functional analyses. Our methodology entails expression of DkTx in E. coli followed by oxidative folding of the isolated linear peptide. Upon screening of various oxidative conditions for optimizing the folding yield of the toxin, we observed that detergents were required for efficient folding of the linear peptide. Our synthetic DkTx co-eluted with the native toxin on HPLC, and irreversibly activated TRPV1 in a manner identical to native DkTx. Interestingly, we find that DkTx has two interconvertible conformations present in a 1∶6 ratio at equilibrium. Kinetic analysis of DkTx folding suggests that the K1 and K2 domains influence each other during the folding process. Moreover, the CD spectra of the toxins shows that the secondary structures of K1 and K2 remains intact even after separating the two knots. These findings provide a starting point for detailed studies on the structural and functional characterization of DkTx and utilization of this toxin as a tool to explore the elusive mechanisms underlying the polymodal gating of TRPV1.
Conflict of interest statement
Figures



References
-
- Isbister GK, Fan HW (2011) Spider bite. Lancet 378: 2039–2047. - PubMed
-
- Adams ME, Herold EE, Venema VJ (1989) Two classes of channel-specific toxins from funnel web spider venom. J Comp Physiol A 164: 333–342. - PubMed
-
- Gomes PC, de Souza BM, Dias NB, Cesar-Tognoli LM, Silva-Filho LC, et al. (2011) Nigriventrine: a low molecular mass neuroactive compound from the venom of the spider Phoneutria nigriventer. Toxicon 57: 266–274. - PubMed
-
- Kitaguchi T, Swartz KJ (2005) An inhibitor of TRPV1 channels isolated from funnel Web spider venom. Biochemistry 44: 15544–15549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

