Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea
- PMID: 23240046
- PMCID: PMC3519890
- DOI: 10.1371/journal.pone.0051574
Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea
Abstract
Macrophages are the primary effector cells of the innate immune system and are also activated in response to tissue injury. The avian cochlea contains a population of resident macrophages, but the precise function of those cells is not known. The present study characterized the behavior of cochlear macrophages after aminoglycoside ototoxicity and also examined the possible role of macrophages in sensory regeneration. We found that the undamaged chick cochlea contains a large resting population of macrophages that reside in the hyaline cell region, immediately outside the abneural (inferior) border of the sensory epithelium. Following ototoxic injury, macrophages appear to migrate out of the hyaline cell region and towards the basilar membrane, congregating immediately below the lesioned sensory epithelium. In order to determine whether recruited macrophages contribute to the regeneration of sensory receptors, we quantified supporting cell proliferation and hair cell recovery after the elimination of most resident macrophages via application of liposomally-encapsulated clodronate. Examination of macrophage-depleted specimens at two days following ototoxic injury revealed no deficits in hair cell clearance, when compared to normal controls. In addition, we found that elimination of macrophages did not affect either regenerative proliferation of supporting cells or the production of replacement hair cells. However, we did find that macrophage-depleted cochleae contained reduced numbers of proliferative mesothelial cells below the basilar membrane. Our data suggest that macrophages are not required for normal debris clearance and regeneration, but that they may play a role in the maintenance of the basilar membrane.
Conflict of interest statement
Figures
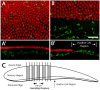
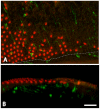




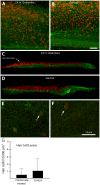


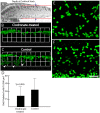

Similar articles
-
Macrophage activity in organ cultures of the avian cochlea: demonstration of a resident population and recruitment to sites of hair cell lesions.J Neurobiol. 1997 Nov 20;33(6):724-34. J Neurobiol. 1997. PMID: 9369147
-
Avian cochlear hair cell regeneration: stereological analyses of damage and recovery from a single high dose of gentamicin.Hear Res. 1995 Dec;92(1-2):17-29. doi: 10.1016/0378-5955(95)00190-5. Hear Res. 1995. PMID: 8647739
-
Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response.J Neurosci. 1996 Sep 1;16(17):5466-77. doi: 10.1523/JNEUROSCI.16-17-05466.1996. J Neurosci. 1996. PMID: 8757259 Free PMC article.
-
Structural recovery from sound and aminoglycoside damage in the avian cochlea.Audiol Neurootol. 1999 Nov-Dec;4(6):271-85. doi: 10.1159/000013852. Audiol Neurootol. 1999. PMID: 10516388 Review.
-
Hair cell regeneration in the avian cochlea.Ann Otol Rhinol Laryngol Suppl. 1997 May;168:9-15. Ann Otol Rhinol Laryngol Suppl. 1997. PMID: 9153111 Review.
Cited by
-
Macrophages gain a partner at the table: epidermal cells digest peripheral dendritic debris in Drosophila.Neuron. 2014 Feb 5;81(3):465-7. doi: 10.1016/j.neuron.2014.01.029. Neuron. 2014. PMID: 24507184 Free PMC article.
-
The Detrimental and Beneficial Functions of Macrophages After Cochlear Injury.Front Cell Dev Biol. 2021 Aug 11;9:631904. doi: 10.3389/fcell.2021.631904. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458249 Free PMC article. Review.
-
Alleviation of skin inflammation after Lin(-) cell transplantation correlates with their differentiation into myeloid-derived suppressor cells.Sci Rep. 2015 Oct 6;5:14663. doi: 10.1038/srep14663. Sci Rep. 2015. PMID: 26441031 Free PMC article.
-
Macrophages Respond Rapidly to Ototoxic Injury of Lateral Line Hair Cells but Are Not Required for Hair Cell Regeneration.Front Cell Neurosci. 2021 Jan 8;14:613246. doi: 10.3389/fncel.2020.613246. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33488362 Free PMC article.
-
Initiation of Supporting Cell Activation for Hair Cell Regeneration in the Avian Auditory Epithelium: An Explant Culture Model.Front Cell Neurosci. 2020 Nov 12;14:583994. doi: 10.3389/fncel.2020.583994. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33281558 Free PMC article.
References
-
- Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11: 1847–1857. - PubMed
-
- Hirose K, Westrum LE, Cunningham DE, Rubel EW (2004) Electron microscopy of degenerative changes in the chick basilar papilla after gentamicin exposure. J Comp Neurol 470: 164–180. - PubMed
-
- Warchol ME (1997) Macrophage activity in the avian cochlea: demonstration of a resident population and recruitment to sites of hair cell lesions. J Neurobiol 33: 724–734. - PubMed
-
- Warchol ME (1999) Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J Neurocytol 28: 889–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

