Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory
- PMID: 23240617
- PMCID: PMC4540228
- DOI: 10.1111/jnc.12130
Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory
Abstract
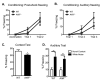
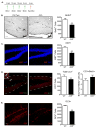
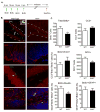
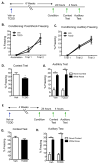
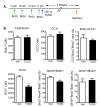
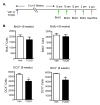
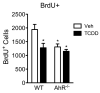
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates the toxicity of dioxin and serves multiple developmental roles. In the adult brain, while we now localize AhR mRNA to nestin-expressing neural progenitor cells in the dentate gyrus (DG) of the hippocampus, its function is unknown. This study tested the hypothesis that AhR participates in hippocampal neurogenesis and associated functions. AhR deletion and activation by the potent environmental toxicant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), adversely impacted neurogenesis and cognition. Adult AhR-deficient mice exhibited impaired hippocampal-dependent contextual fear memory while hippocampal-independent memory remained intact. AhR-deficient mice displayed reduced cell birth, decreased cell survival, and diminished neuronal differentiation in the DG. Following TCDD exposure, wild-type mice exhibited impaired hippocampal-dependent contextual memory, decreased cell birth, reduced neuronal differentiation, and fewer mature neurons in the DG. Glial differentiation and apoptosis were not altered in either TCDD-exposed or AhR-deficient mice. Finally, defects observed in TCDD-exposed mice were dependent on AhR, as TCDD had no negative effects in AhR-deficient mice. Our findings suggest that AhR should be further evaluated as a potential transcriptional regulator of hippocampal neurogenesis and function, although other sites of action may also warrant consideration. Moreover, TCDD exposure should be considered as an environmental risk factor that disrupts adult neurogenesis and potentially related memory processes.
© 2012 International Society for Neurochemistry.
Conflict of interest statement
No conflicts of interests exist.
Figures


Similar articles
-
Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory.J Neurosci. 2011 Jul 6;31(27):9933-44. doi: 10.1523/JNEUROSCI.1062-11.2011. J Neurosci. 2011. PMID: 21734285 Free PMC article.
-
Neural precursor cell proliferation is disrupted through activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin.Stem Cells Dev. 2011 Feb;20(2):313-26. doi: 10.1089/scd.2009.0529. Epub 2010 Aug 31. Stem Cells Dev. 2011. PMID: 20486776 Free PMC article.
-
Posttraining ablation of adult-generated neurons degrades previously acquired memories.J Neurosci. 2011 Oct 19;31(42):15113-27. doi: 10.1523/JNEUROSCI.3432-11.2011. J Neurosci. 2011. PMID: 22016545 Free PMC article.
-
The Aryl Hydrocarbon Receptor in Energy Balance: The Road from Dioxin-Induced Wasting Syndrome to Combating Obesity with Ahr Ligands.Int J Mol Sci. 2020 Dec 23;22(1):49. doi: 10.3390/ijms22010049. Int J Mol Sci. 2020. PMID: 33374508 Free PMC article. Review.
-
AHR-mediated immunomodulation: the role of altered gene transcription.Biochem Pharmacol. 2009 Feb 15;77(4):746-60. doi: 10.1016/j.bcp.2008.11.021. Epub 2008 Nov 27. Biochem Pharmacol. 2009. PMID: 19100241 Free PMC article. Review.
Cited by
-
Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective.Front Integr Neurosci. 2020 Aug 5;14:44. doi: 10.3389/fnint.2020.00044. eCollection 2020. Front Integr Neurosci. 2020. PMID: 32848651 Free PMC article. Review.
-
The Aryl Hydrocarbon Receptor: A Mediator and Potential Therapeutic Target for Ocular and Non-Ocular Neurodegenerative Diseases.Int J Mol Sci. 2020 Sep 16;21(18):6777. doi: 10.3390/ijms21186777. Int J Mol Sci. 2020. PMID: 32947781 Free PMC article. Review.
-
Gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin primes cortical microglia to tissue injury.Brain Behav Immun. 2022 Mar;101:288-303. doi: 10.1016/j.bbi.2022.01.013. Epub 2022 Jan 19. Brain Behav Immun. 2022. PMID: 35065196 Free PMC article.
-
Aryl hydrocarbon receptor downregulates MYCN expression and promotes cell differentiation of neuroblastoma.PLoS One. 2014 Feb 21;9(2):e88795. doi: 10.1371/journal.pone.0088795. eCollection 2014. PLoS One. 2014. PMID: 24586395 Free PMC article.
-
Selective Aryl Hydrocarbon Receptor Modulator 3,3'-Diindolylmethane Impairs AhR and ARNT Signaling and Protects Mouse Neuronal Cells Against Hypoxia.Mol Neurobiol. 2016 Oct;53(8):5591-606. doi: 10.1007/s12035-015-9471-0. Epub 2015 Oct 17. Mol Neurobiol. 2016. PMID: 26476840
References
-
- Aasebo IE, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. Eur J Neurosci. 2011;33:1094–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

