Regulating the ARNT/TACC3 axis: multiple approaches to manipulating protein/protein interactions with small molecules
- PMID: 23240775
- PMCID: PMC3600089
- DOI: 10.1021/cb300604u
Regulating the ARNT/TACC3 axis: multiple approaches to manipulating protein/protein interactions with small molecules
Abstract
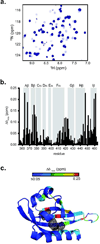
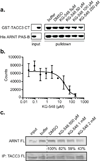
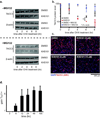
For several well-documented reasons, it has been challenging to develop artificial small molecule inhibitors of protein/protein complexes. Such reagents are of particular interest for transcription factor complexes given links between their misregulation and disease. Here we report parallel approaches to identify regulators of a hypoxia signaling transcription factor complex, involving the ARNT subunit of the HIF (Hypoxia Inducible Factor) activator and the TACC3 (Transforming Acidic Coiled Coil Containing Protein 3) coactivator. In one route, we used in vitro NMR and biochemical screening to identify small molecules that selectively bind within the ARNT PAS (Per-ARNT-Sim) domain that recruits TACC3, identifying KG-548 as an ARNT/TACC3 disruptor. A parallel, cell-based screening approach previously implicated the small molecule KHS101 as an inhibitor of TACC3 signaling. Here, we show that KHS101 works indirectly on HIF complex formation by destabilizing both TACC3 and the HIF component HIF-1α. Overall, our data identify small molecule regulators for this important complex and highlight the utility of pursuing parallel strategies to develop protein/protein inhibitors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

