Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle
- PMID: 23241324
- PMCID: PMC3566483
- DOI: 10.1152/ajpheart.00754.2012
Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle
Abstract
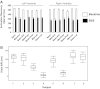
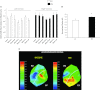
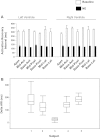
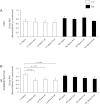
Increased cardiac sympathetic activation worsens dispersion of repolarization and is proarrhythmic. The functional differences between intrinsic nerve stimulation and adrenergic receptor activation remain incompletely understood. This study was undertaken to determine the functional differences between efferent cardiac sympathetic nerve stimulation and direct adrenergic receptor activation in porcine ventricles. Female Yorkshire pigs (n = 13) underwent surgical exposure of the heart and stellate ganglia. A 56-electrode sock was placed over the ventricles to record epicardial electrograms. Animals underwent bilateral sympathetic stimulation (BSS) (n = 8) or norepinephrine (NE) administration (n = 5). Activation recovery intervals (ARIs) were measured at each electrode before and during BSS or NE. The degree of ARI shortening during BSS or NE administration was used as a measure of functional nerve or adrenergic receptor density. During BSS, ARI shortening was nonuniform across the epicardium (F value 9.62, P = 0.003), with ARI shortening greatest in the mid-basal lateral right ventricle and least in the midposterior left ventricle (LV) (mean normalized values: 0.9 ± 0.08 vs. 0.56 ± 0.08; P = 0.03). NE administration resulted in greater ARI shortening in the LV apex than basal segments [0.91 ± 0.04 vs. 0.63 ± 0.05 (averaged basal segments); P = 0.003]. Dispersion of ARIs increased in 50% and 60% of the subjects undergoing BSS and NE, respectively, but decreased in the others. There is nonuniform response to cardiac sympathetic activation of both porcine ventricles, which is not fully explained by adrenergic receptor density. Different pools of adrenergic receptors may mediate the cardiac electrophysiological effects of efferent sympathetic nerve activity and circulating catecholamines.
Figures





References
-
- Amerini S, Rubino A, Filippi S, Ledda F, Mantelli L. Modulation by adrenergic transmitters of the efferent function of capsaicin-sensitive nerves in cardiac tissue. Neuropeptides 20: 225–232, 1991 - PubMed
-
- Angelakos ET. Regional distribution of catecholamines in the dog heart. Circ Res 16: 39–44, 1965 - PubMed
-
- Angelakos ET, Fuxe K, Torchiana ML. Chemical and histochemical evaluation of the distribution of catecholamines in the rabbit and guinea pig hearts. Acta physiologica Scandinavica 59: 184–192, 1963 - PubMed
-
- Basu S, Sinha SK, Shao Q, Ganguly PK, Dhalla NS. Neuropeptide Y modulation of sympathetic activity in myocardial infarction. Journal of the American College of Cardiology 27: 1796–1803, 1996 - PubMed
-
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

