Analysis of alternative signaling pathways of endoderm induction of human embryonic stem cells identifies context specific differences
- PMID: 23241383
- PMCID: PMC3547704
- DOI: 10.1186/1752-0509-6-154
Analysis of alternative signaling pathways of endoderm induction of human embryonic stem cells identifies context specific differences
Abstract
Background: Lineage specific differentiation of human embryonic stem cells (hESCs) is largely mediated by specific growth factors and extracellular matrix molecules. Growth factors initiate a cascade of signals which control gene transcription and cell fate specification. There is a lot of interest in inducing hESCs to an endoderm fate which serves as a pathway towards more functional cell types like the pancreatic cells. Research over the past decade has established several robust pathways for deriving endoderm from hESCs, with the capability of further maturation. However, in our experience, the functional maturity of these endoderm derivatives, specifically to pancreatic lineage, largely depends on specific pathway of endoderm induction. Hence it will be of interest to understand the underlying mechanism mediating such induction and how it is translated to further maturation. In this work we analyze the regulatory interactions mediating different pathways of endoderm induction by identifying co-regulated transcription factors.
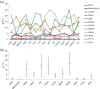
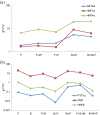
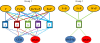
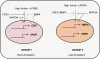
Results: hESCs were induced towards endoderm using activin A and 4 different growth factors (FGF2 (F), BMP4 (B), PI3KI (P), and WNT3A (W)) and their combinations thereof, resulting in 15 total experimental conditions. At the end of differentiation each condition was analyzed by qRT-PCR for 12 relevant endoderm related transcription factors (TFs). As a first approach, we used hierarchical clustering to identify which growth factor combinations favor up-regulation of different genes. In the next step we identified sets of co-regulated transcription factors using a biclustering algorithm. The high variability of experimental data was addressed by integrating the biclustering formulation with bootstrap re-sampling to identify robust networks of co-regulated transcription factors. Our results show that the transition from early to late endoderm is favored by FGF2 as well as WNT3A treatments under high activin. However, induction of late endoderm markers is relatively favored by WNT3A under high activin.
Conclusions: Use of FGF2, WNT3A or PI3K inhibition with high activin A may serve well in definitive endoderm induction followed by WNT3A specific signaling to direct the definitive endoderm into late endodermal lineages. Other combinations, though still feasible for endoderm induction, appear less promising for pancreatic endoderm specification in our experiments.
Figures



Similar articles
-
FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner.PLoS One. 2009;4(3):e4794. doi: 10.1371/journal.pone.0004794. Epub 2009 Mar 11. PLoS One. 2009. PMID: 19277121 Free PMC article.
-
Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells.Mech Dev. 2011 Sep-Dec;128(7-10):412-27. doi: 10.1016/j.mod.2011.08.001. Epub 2011 Aug 10. Mech Dev. 2011. PMID: 21855631 Free PMC article.
-
Efficient Definitive Endoderm Differentiation from Human Parthenogenetic Embryonic Stem Cells Induced by Activin A and Wnt3a.Ann Clin Lab Sci. 2020 Jul;50(4):468-473. Ann Clin Lab Sci. 2020. PMID: 32826243
-
Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors-the influence of differentiation factors and culture conditions.Stem Cell Rev Rep. 2009 Jun;5(2):159-73. doi: 10.1007/s12015-009-9061-5. Epub 2009 Mar 5. Stem Cell Rev Rep. 2009. PMID: 19263252 Review.
-
Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm.Cold Spring Harb Symp Quant Biol. 2008;73:119-26. doi: 10.1101/sqb.2008.73.040. Epub 2008 Nov 21. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19028990 Free PMC article. Review.
Cited by
-
Quantitative Analysis of Robustness of Dynamic Response and Signal Transfer in Insulin mediated PI3K/AKT Pathway.Comput Chem Eng. 2014 Dec 4;71:715-727. doi: 10.1016/j.compchemeng.2014.07.018. Comput Chem Eng. 2014. PMID: 25506104 Free PMC article.
-
Signals from the surface modulate differentiation of human pluripotent stem cells through glycosaminoglycans and integrins.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18126-31. doi: 10.1073/pnas.1409525111. Epub 2014 Nov 24. Proc Natl Acad Sci U S A. 2014. PMID: 25422477 Free PMC article.
-
Potential for pancreatic maturation of differentiating human embryonic stem cells is sensitive to the specific pathway of definitive endoderm commitment.PLoS One. 2014 Apr 17;9(4):e94307. doi: 10.1371/journal.pone.0094307. eCollection 2014. PLoS One. 2014. PMID: 24743345 Free PMC article.
-
Endothelial cells mediate islet-specific maturation of human embryonic stem cell-derived pancreatic progenitor cells.Tissue Eng Part A. 2015 Jan;21(1-2):14-25. doi: 10.1089/ten.TEA.2014.0013. Epub 2014 Aug 19. Tissue Eng Part A. 2015. PMID: 24943736 Free PMC article.
-
Differentiation of Definitive Endoderm from Human Induced Pluripotent Stem Cells on hMSCs Feeder in a Defined Medium.Avicenna J Med Biotechnol. 2016 Jan-Mar;8(1):2-8. Avicenna J Med Biotechnol. 2016. PMID: 26855729 Free PMC article.
References
-
- Payne C, King J, Hay D. The role of activin/nodal and Wnt signaling in endoderm formation. Activins Inhibins. 2011;85:207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

