Association of telomere instability with senescence of porcine cells
- PMID: 23241441
- PMCID: PMC3563453
- DOI: 10.1186/1471-2121-13-36
Association of telomere instability with senescence of porcine cells
Abstract
Background: Telomeres are essential for the maintenance of genomic stability, and telomere dysfunction leads to cellular senescence, carcinogenesis, aging, and age-related diseases in humans. Pigs have become increasingly important large animal models for preclinical tests and study of human diseases, and also may provide xeno-transplantation sources. Thus far, Southern blot analysis has been used to estimate average telomere lengths in pigs. Telomere quantitative fluorescence in situ hybridization (Q-FISH), however, can reveal status of individual telomeres in fewer cells, in addition to quantifying relative telomere lengths, and has been commonly used for study of telomere function of mouse and human cells. We attempted to investigate telomere characteristics of porcine cells using telomere Q-FISH method.
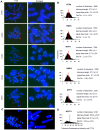
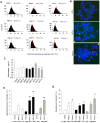
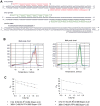
Results: The average telomere lengths in porcine cells measured by Q-FISH correlated with those of quantitative real-time PCR method (qPCR) or telomere restriction fragments (TRFs) by Southern blot analysis. Unexpectedly, we found that porcine cells exhibited high incidence of telomere doublets revealed by Q-FISH method, coincided with increased frequency of cellular senescence. Also, telomeres shortened during subculture of various porcine primary cell types. Interestingly, the high frequency of porcine telomere doublets and telomere loss was associated with telomere dysfunction-induced foci (TIFs). The incidence of TIFs, telomere doublets and telomere loss increased with telomere shortening and cellular senescence during subculture.
Conclusion: Q-FISH method using telomere PNA probe is particularly useful for characterization of porcine telomeres. Porcine cells exhibit high frequency of telomere instability and are susceptible to telomere damage and replicative senescence.
Figures

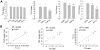
Similar articles
-
Comparison of telomere length measurement methods.Philos Trans R Soc Lond B Biol Sci. 2018 Mar 5;373(1741):20160451. doi: 10.1098/rstb.2016.0451. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29335378 Free PMC article. Review.
-
Telomere Length Measurement in Human Tissue Sections by Quantitative Fluorescence In Situ Hybridization (Q-FISH).Methods Mol Biol. 2025;2857:9-14. doi: 10.1007/978-1-0716-4128-6_2. Methods Mol Biol. 2025. PMID: 39348051
-
Autophagy-independent senescence and genome instability driven by targeted telomere dysfunction.Autophagy. 2015;11(3):527-37. doi: 10.1080/15548627.2015.1017189. Autophagy. 2015. PMID: 25751002 Free PMC article.
-
Human Xp/Yp telomere analysis by Southern-STELA.Biogerontology. 2012 Apr;13(2):203-13. doi: 10.1007/s10522-011-9368-x. Epub 2011 Dec 6. Biogerontology. 2012. PMID: 22143823
-
Repair of telomeric DNA prior to replicative senescence.Mech Ageing Dev. 2000 Sep 1;118(1-2):23-34. doi: 10.1016/s0047-6374(00)00151-2. Mech Ageing Dev. 2000. PMID: 10989122 Review.
Cited by
-
Genome-wide DNA methylation changes in skeletal muscle between young and middle-aged pigs.BMC Genomics. 2014 Aug 5;15(1):653. doi: 10.1186/1471-2164-15-653. BMC Genomics. 2014. PMID: 25096499 Free PMC article.
-
DNA repair and replication links to pluripotency and differentiation capacity of pig iPS cells.PLoS One. 2017 Mar 2;12(3):e0173047. doi: 10.1371/journal.pone.0173047. eCollection 2017. PLoS One. 2017. PMID: 28253351 Free PMC article.
-
Human MLH1 suppresses the insertion of telomeric sequences at intra-chromosomal sites in telomerase-expressing cells.Nucleic Acids Res. 2017 Feb 17;45(3):1219-1232. doi: 10.1093/nar/gkw1170. Nucleic Acids Res. 2017. PMID: 28180301 Free PMC article.
-
p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle.Elife. 2020 Mar 3;9:e52570. doi: 10.7554/eLife.52570. Elife. 2020. PMID: 32125276 Free PMC article.
-
Telomere elongation facilitated by trichostatin a in cloned embryos and pigs by somatic cell nuclear transfer.Stem Cell Rev Rep. 2014 Jun;10(3):399-407. doi: 10.1007/s12015-014-9499-y. Stem Cell Rev Rep. 2014. PMID: 24510582
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

