The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection
- PMID: 23241878
- PMCID: PMC3879798
- DOI: 10.4049/jimmunol.1202448
The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection
Abstract
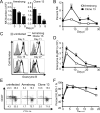
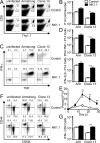
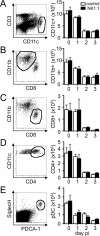
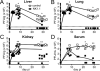
NK cells have well-established functions in immune defense against virus infections and cancer through their cytolytic activity and production of cytokines. In this study, we examined the frequency of NK cells and their influence on T cell responses in mice given variants of lymphocytic choriomeningitis virus that cause acute or persisting infection. We found increased frequencies of circulating NK cells during disseminating infection compared with uninfected or acutely infected mice. Consistent with recent reports, we observed that the depletion of NK cells in mice with disseminated infection increased peak numbers of virus-specific cytokine producing CD8(+) T cells and resulted in the rapid resolution of disseminated infection. Additionally, we show that NK cell depletion sustained T cell responses across time and protected against T cell exhaustion. The positive effects of NK cell depletion on T cell responses only occurred when NK cells were depleted within the first 2 d of infection. We find that the improved CD8(+) T cell response correlated with an enhanced ability of APCs from NK cell-depleted mice to stimulate T cell proliferation, independently of the effects of NK cells on CD4(+) T cells. These results indicate that NK cells play an integral role in limiting the CD8 T cell response and contribute to T cell exhaustion by diminishing APC function during persisting virus infection.
Figures



References
-
- Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. - PubMed
-
- Seich Al Basatena NK, Macnamara A, Vine AM, Thio CL, Astemborski J, Usuku K, Osame M, Kirk GD, Donfield SM, Goedert JJ, Bangham CR, Carrington M, Khakoo SI, Asquith B. KIR2DL2 enhances protective and detrimental HLA class I-mediated immunity in chronic viral infection. PLoS pathogens. 2011;7:e1002270. - PMC - PubMed
-
- Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. - PubMed
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual review of immunology. 1999;17:189–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

