Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair
- PMID: 23241962
- PMCID: PMC3533303
- DOI: 10.1172/JCI65831
Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair
Abstract
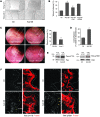
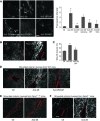
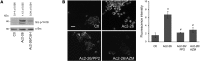
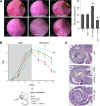
N-formyl peptide receptors (FPRs) are critical regulators of host defense in phagocytes and are also expressed in epithelia. FPR signaling and function have been extensively studied in phagocytes, yet their functional biology in epithelia is poorly understood. We describe a novel intestinal epithelial FPR signaling pathway that is activated by an endogenous FPR ligand, annexin A1 (ANXA1), and its cleavage product Ac2-26, which mediate activation of ROS by an epithelial NADPH oxidase, NOX1. We show that epithelial cell migration was regulated by this signaling cascade through oxidative inactivation of the regulatory phosphatases PTEN and PTP-PEST, with consequent activation of focal adhesion kinase (FAK) and paxillin. In vivo studies using intestinal epithelial specific Nox1(-/-IEC) and AnxA1(-/-) mice demonstrated defects in intestinal mucosal wound repair, while systemic administration of ANXA1 promoted wound recovery in a NOX1-dependent fashion. Additionally, increased ANXA1 expression was observed in the intestinal epithelium and infiltrating leukocytes in the mucosa of ulcerative colitis patients compared with normal intestinal mucosa. Our findings delineate a novel epithelial FPR1/NOX1-dependent redox signaling pathway that promotes mucosal wound repair.
Figures



Similar articles
-
Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1.Mucosal Immunol. 2014 May;7(3):645-55. doi: 10.1038/mi.2013.84. Epub 2013 Nov 6. Mucosal Immunol. 2014. PMID: 24192910 Free PMC article.
-
NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes.J Immunol. 2003 Jul 1;171(1):299-306. doi: 10.4049/jimmunol.171.1.299. J Immunol. 2003. PMID: 12817011
-
NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon.Mol Cell Biol. 2010 Jun;30(11):2636-50. doi: 10.1128/MCB.01194-09. Epub 2010 Mar 29. Mol Cell Biol. 2010. PMID: 20351171 Free PMC article.
-
Redox signaling mediated by the gut microbiota.Free Radic Biol Med. 2017 Apr;105:41-47. doi: 10.1016/j.freeradbiomed.2016.10.495. Epub 2016 Oct 29. Free Radic Biol Med. 2017. PMID: 27989756 Review.
-
Redox signaling mediated by the gut microbiota.Free Radic Res. 2013 Nov;47(11):950-7. doi: 10.3109/10715762.2013.833331. Epub 2013 Oct 4. Free Radic Res. 2013. PMID: 23937589 Free PMC article. Review.
Cited by
-
UEG Week 2020 Poster Presentations.United European Gastroenterol J. 2020 Oct;8(8_suppl):144-887. doi: 10.1177/2050640620927345. United European Gastroenterol J. 2020. PMID: 33043826 Free PMC article. No abstract available.
-
Intrinsic Defense Mechanisms of the Intestinal Epithelium.Cell Host Microbe. 2016 Apr 13;19(4):434-41. doi: 10.1016/j.chom.2016.03.003. Epub 2016 Mar 31. Cell Host Microbe. 2016. PMID: 27049583 Free PMC article. Review.
-
Wound repair: role of immune-epithelial interactions.Mucosal Immunol. 2015 Sep;8(5):959-68. doi: 10.1038/mi.2015.63. Epub 2015 Jul 15. Mucosal Immunol. 2015. PMID: 26174765 Free PMC article. Review.
-
Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway.Cell Rep. 2015 Aug 25;12(8):1217-25. doi: 10.1016/j.celrep.2015.07.042. Epub 2015 Aug 13. Cell Rep. 2015. PMID: 26279578 Free PMC article.
-
Impact of Epithelial Cell Shedding on Intestinal Homeostasis.Int J Mol Sci. 2022 Apr 9;23(8):4160. doi: 10.3390/ijms23084160. Int J Mol Sci. 2022. PMID: 35456978 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK072564/DK/NIDDK NIH HHS/United States
- R01 DK079392/DK/NIDDK NIH HHS/United States
- R01DK089763/DK/NIDDK NIH HHS/United States
- R01HL096796-3/HL/NHLBI NIH HHS/United States
- DK079392/DK/NIDDK NIH HHS/United States
- DK061379/DK/NIDDK NIH HHS/United States
- R01 DK072564/DK/NIDDK NIH HHS/United States
- R01AI64462/AI/NIAID NIH HHS/United States
- R01 DK055679/DK/NIDDK NIH HHS/United States
- R01DK055679/DK/NIDDK NIH HHS/United States
- R01 DK061379/DK/NIDDK NIH HHS/United States
- R01 AI064462/AI/NIAID NIH HHS/United States
- R01 HL096796/HL/NHLBI NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK089763/DK/NIDDK NIH HHS/United States
- DK064399/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

