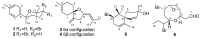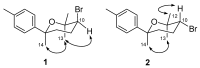Sesquiterpene and acetogenin derivatives from the marine red alga Laurencia okamurai
- PMID: 23242203
- PMCID: PMC3528128
- DOI: 10.3390/md10122817
Sesquiterpene and acetogenin derivatives from the marine red alga Laurencia okamurai
Abstract
In addition to 13 known compounds, four new bisabolane sesquiterpenes, okamurenes A-D (1-4), a new chamigrane derivative, okamurene E (5), and a new C₁₂-acetogenin, okamuragenin (6), were isolated from the marine red alga Laurencia okamurai. The structures of these compounds were determined through detailed spectroscopic analyses. Of these, okamurenes A and B (1 and 2) are the first examples of bromobisabolane sesquiterpenes possessing a phenyl moiety among Laurencia-derived sesquiterpenes, while okamuragenin (6) was the first acetogenin aldehyde possessing a C₁₂-carbon skeleton. Each of the isolated compounds was evaluated for the brine shrimp (Artemia salina) lethal assay and 7-hydroxylaurene displayed potent lethality with LD₅₀ 1.8 μM.
Figures
Similar articles
-
Sesquiterpenes and acetogenins from the marine red alga Laurencia okamurai.Fitoterapia. 2012 Apr;83(3):518-22. doi: 10.1016/j.fitote.2011.12.018. Epub 2011 Dec 30. Fitoterapia. 2012. PMID: 22233863
-
Laurane-, cyclolaurane-, and cuparane-type sesquiterpenes from the marine red alga Laurencia okamurai.Nat Prod Commun. 2014 Mar;9(3):323-4. Nat Prod Commun. 2014. PMID: 24689206
-
Diterpenes, sesquiterpenes, and a C15-acetogenin from the marine red alga Laurencia mariannensis.J Nat Prod. 2007 Dec;70(12):1901-5. doi: 10.1021/np070378b. Epub 2007 Dec 13. J Nat Prod. 2007. PMID: 18076141
-
Sesquiterpenes from the marine red alga Laurencia composita.Fitoterapia. 2012 Oct;83(7):1191-5. doi: 10.1016/j.fitote.2012.07.001. Epub 2012 Jul 14. Fitoterapia. 2012. PMID: 22796401
-
The Laurencia Paradox: An Endless Source of Chemodiversity.Prog Chem Org Nat Prod. 2016;102:91-252. doi: 10.1007/978-3-319-33172-0_2. Prog Chem Org Nat Prod. 2016. PMID: 27380407 Review.
Cited by
-
Additional evidence of the trypanocidal action of (-)-elatol on amastigote forms through the involvement of reactive oxygen species.Mar Drugs. 2014 Sep 25;12(9):4973-83. doi: 10.3390/md12094973. Mar Drugs. 2014. PMID: 25257785 Free PMC article.
-
Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases.Molecules. 2018 Nov 20;23(11):3032. doi: 10.3390/molecules23113032. Molecules. 2018. PMID: 30463364 Free PMC article.
-
Chemistry and Bioactivity of Marine-Derived Bisabolane Sesquiterpenoids: A Review.Front Chem. 2022 Apr 7;10:881767. doi: 10.3389/fchem.2022.881767. eCollection 2022. Front Chem. 2022. PMID: 35464222 Free PMC article. Review.
-
Biological Activity of Recently Discovered Halogenated Marine Natural Products.Mar Drugs. 2015 Jun 30;13(7):4044-136. doi: 10.3390/md13074044. Mar Drugs. 2015. PMID: 26133553 Free PMC article. Review.
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
References
-
- Ji N.-Y., Li X.-M., Zhang Y., Wang B.-G. Two new halogenated chamigrane-type sesquiterpenes and other secondary metabolites from the marine red alga Laurencia okamurai and their chemotaxonomic significance. Biochem. Syst. Ecol. 2007;35:627–630. doi: 10.1016/j.bse.2007.02.004. - DOI
-
- Sugimoto M., Suzuki T., Hagiwara H., Hoshi T. The first total synthesis of (+)-(Z)-laureatin. Tetrahedron Lett. 2007;48:1109–1112. doi: 10.1016/j.tetlet.2006.12.082. - DOI
-
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 1996;13:75–125. - PubMed
-
- Masuda M., Abe T., Suzuki T., Suzuki M. Morphological and chemotaxonomic studies on Laurencia composita and L. okamurae (Ceramiales, Rhodophyta) Phycologia . 1996;35:550–562. doi: 10.2216/i0031-8884-35-6-550.1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources