Use of the Syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers
- PMID: 23242370
- PMCID: PMC3528289
- DOI: 10.3390/v4123754
Use of the Syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers
Abstract
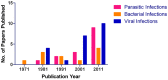
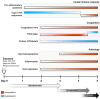
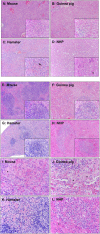
Historically, mice and guinea pigs have been the rodent models of choice for therapeutic and prophylactic countermeasure testing against Ebola virus disease (EVD). Recently, hamsters have emerged as a novel animal model for the in vivo study of EVD. In this review, we discuss the history of the hamster as a research laboratory animal, as well as current benefits and challenges of this model. Availability of immunological reagents is addressed. Salient features of EVD in hamsters, including relevant pathology and coagulation parameters, are compared directly with the mouse, guinea pig and nonhuman primate models.
Figures
References
-
- Schnittler H.J., Feldmann H. Viral hemorrhagic fever--a vascular disease? Thromb. Haemostasis. 2003;89:967–972. - PubMed
-
- Zaki S.R., Goldsmith C.S. Pathologic features of filovirus infections in humans. Curr. Top. Microbiol. Immunol. 1999;235:97–116. - PubMed
-
- Bwaka M.A., Bonnet M.J., Calain P., Colebunders R., De Roo A., Guimard Y., Katwiki K.R., Kibadi K., Kipasa M.A., Kuvula K.J., et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 1999;179:S1–S7. doi: 10.1086/314568. - DOI - PubMed
-
- Formenty P., Hatz C., Le Guenno B., Stoll A., Rogenmoser P., Widmer A. Human infection due to Ebola virus, subtype Cote d'Ivoire: Clinical and biologic presentation. J. Infect. Dis. 1999;179:S48–S53. - PubMed
-
- Kuhn J.H. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch. Virol. Suppl. 2008;20:13–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

