The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood
- PMID: 23242415
- PMCID: PMC3788614
- DOI: 10.1038/ni.2496
The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood
Abstract
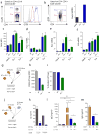
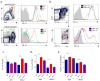
CD4(+)CXCR5(+)Foxp3(+) follicular regulatory T cells (T(FR) cells) inhibit humoral immunity mediated by CD4(+)CXCR5(+)Foxp3(-) follicular helper T cells (T(FH) cells). Although the inhibitory receptor PD-1 is expressed by both cell types, its role in the differentiation of T(FR) cells is unknown. Here we found that mice deficient in PD-1 and its ligand PD-L1 had a greater abundance of T(FR) cells in the lymph nodes and that those T(FR) cells had enhanced suppressive ability. We also found substantial populations of T(FR) cells in mouse blood and demonstrated that T(FR) cells in the blood homed to lymph nodes and potently inhibited T(FH) cells in vivo. T(FR) cells in the blood required signaling via the costimulatory receptors CD28 and ICOS but were inhibited by PD-1 and PD-L1. Our findings demonstrate mechanisms by which the PD-1 pathway regulates antibody production and help reconcile inconsistencies surrounding the role of this pathway in humoral immunity.
Figures






Comment in
-
Critical role of PD-1/PD-L1 pathway in generation and function of follicular regulatory T cells.Cell Mol Immunol. 2013 Jul;10(4):286-8. doi: 10.1038/cmi.2013.15. Epub 2013 Apr 29. Cell Mol Immunol. 2013. PMID: 23624877 Free PMC article. No abstract available.
References
-
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

