How to form and close the brain: insight into the mechanism of cranial neural tube closure in mammals
- PMID: 23242429
- PMCID: PMC3742426
- DOI: 10.1007/s00018-012-1227-7
How to form and close the brain: insight into the mechanism of cranial neural tube closure in mammals
Abstract
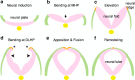
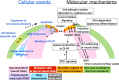
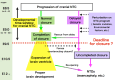
The development of the embryonic brain critically depends on successfully completing cranial neural tube closure (NTC). Failure to properly close the neural tube results in significant and potentially lethal neural tube defects (NTDs). We believe these malformations are caused by disruptions in normal developmental programs such as those involved in neural plate morphogenesis and patterning, tissue fusion, and coordinated cell behaviors. Cranial NTDs include anencephaly and craniorachischisis, both lethal human birth defects. Newly emerging methods for molecular and cellular analysis offer a deeper understanding of not only the developmental NTC program itself but also mechanical and kinetic aspects of closure that may contribute to cranial NTDs. Clarifying the underlying mechanisms involved in NTC and how they relate to the onset of specific NTDs in various experimental models may help us develop novel intervention strategies to prevent NTDs.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

