Plasminogen activator inhibitor 1 RNA-binding protein interacts with progesterone receptor membrane component 1 to regulate progesterone's ability to maintain the viability of spontaneously immortalized granulosa cells and rat granulosa cells
- PMID: 23242527
- PMCID: PMC4434940
- DOI: 10.1095/biolreprod.112.103036
Plasminogen activator inhibitor 1 RNA-binding protein interacts with progesterone receptor membrane component 1 to regulate progesterone's ability to maintain the viability of spontaneously immortalized granulosa cells and rat granulosa cells
Abstract
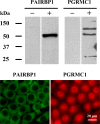
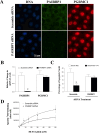
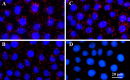
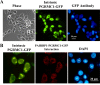
Progesterone receptor membrane component 1 (PGRMC1) mediates the antiapoptotic action of progesterone (P4). PGRMC1 interacts with plasminogen activator inhibitor 1 RNA-binding protein (PAIRBP1), but the functional significance of this interaction is unknown. To examine the function of PGRMC1-PAIRBP1 interaction, PAIRBP1 was depleted from spontaneously immortalized granulosa cells (SIGCs) and the effects on the expression and localization of PGRMC1 as well as P4's ability to bind to SIGCs and prevent apoptosis was assessed. Depleting PAIRBP1 enhanced cellular (3)H-P4 binding and did not alter the expression or cellular localization of PGRMC1 but attenuated P4's antiapoptotic action. Transfection of a PGRMC1-green fluorescent protein (GFP) peptide mimic, which binds PAIRBP1 as demonstrated by in situ proximity assay, doubled the rate at which SIGCs undergo apoptosis compared to cells transfected with either the empty GFP expression vector or Pairbp1 small interfering RNA. Moreover, P4 did not prevent these cells from undergoing apoptosis. Similar studies conducted with granulosa cells isolated from immature rats also showed that PGRMC1 interacts with PAIRBP1 and that transfection of PGRMC1-GFP peptide mimic accelerates the rate of granulosa cell apoptosis by 4-fold even in the presence of serum and P4. These studies support the concept that the interaction between PAIRBP1-PGRMC1 is an essential component of the mechanism through which P4 inhibits apoptosis. Surprisingly, PGRMC1-PAIRBP1 interaction is not required for P4 binding or the cellular localization of PGRMC1 but rather appears to couple PGRMC1 to downstream components of the P4-PGRMC1 signal transduction pathway.
Figures
Similar articles
-
Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations.Endocrinology. 2008 Feb;149(2):534-43. doi: 10.1210/en.2007-1050. Epub 2007 Nov 8. Endocrinology. 2008. PMID: 17991724 Free PMC article.
-
Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action.Endocrinology. 2006 Jun;147(6):3133-40. doi: 10.1210/en.2006-0114. Epub 2006 Mar 2. Endocrinology. 2006. PMID: 16513825
-
Expression and function of PAIRBP1 within gonadotropin-primed immature rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability.Biol Reprod. 2005 Aug;73(2):261-70. doi: 10.1095/biolreprod.105.041061. Epub 2005 Apr 6. Biol Reprod. 2005. PMID: 15814896
-
Non-canonical progesterone signaling in granulosa cell function.Reproduction. 2014 Apr 8;147(5):R169-78. doi: 10.1530/REP-13-0582. Print 2014 May. Reproduction. 2014. PMID: 24516175 Free PMC article. Review.
-
Progesterone as a regulator of granulosa cell viability.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):167-73. doi: 10.1016/s0960-0760(03)00192-4. J Steroid Biochem Mol Biol. 2003. PMID: 12943701 Review.
Cited by
-
Progesterone Signaling and Mammalian Ovarian Follicle Growth Mediated by Progesterone Receptor Membrane Component Family Members.Cells. 2022 May 13;11(10):1632. doi: 10.3390/cells11101632. Cells. 2022. PMID: 35626669 Free PMC article. Review.
-
The HMGA2-IMP2 Pathway Promotes Granulosa Cell Proliferation in Polycystic Ovary Syndrome.J Clin Endocrinol Metab. 2019 Apr 1;104(4):1049-1059. doi: 10.1210/jc.2018-00544. J Clin Endocrinol Metab. 2019. PMID: 30247605 Free PMC article.
-
The RNA-binding protein SERBP1 interacts selectively with the signaling protein RACK1.Cell Signal. 2017 Jul;35:256-263. doi: 10.1016/j.cellsig.2017.03.001. Epub 2017 Mar 4. Cell Signal. 2017. PMID: 28267599 Free PMC article.
-
ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats.Cell Death Dis. 2017 Oct 26;8(10):e3145. doi: 10.1038/cddis.2017.494. Cell Death Dis. 2017. PMID: 29072679 Free PMC article.
-
Expression of progesterone receptor membrane component-2 within the immature rat ovary and its role in regulating mitosis and apoptosis of spontaneously immortalized granulosa cells.Biol Reprod. 2014 Aug;91(2):36. doi: 10.1095/biolreprod.114.117481. Epub 2014 Jul 2. Biol Reprod. 2014. PMID: 24990806 Free PMC article.
References
-
- Fujii T, Hoover DJ, Channing CP. Changes in inhibin activity, and progesterone, oestrogen and androstenedione concentrations, in rat follicular fluid throughout the oestrous cycle. J Reprod Fertil. 1983;69:307–314. - PubMed
-
- Luciano AM, Pappalardo A, Ray C, Peluso JJ. Epidermal growth factor inhibits large granulosa cell apoptosis by stimulating progesterone synthesis and regulating the distribution of intracellular free calcium. Biol Reprod. 1994;51:646–654. - PubMed
-
- Luciano AM, Peluso JJ. Effect of in vivo gonadotropin treatment on the ability of progesterone, estrogen, and cyclic adenosine 5′-monophosphate to inhibit insulin-dependent granulosa cell mitosis in vitro. Biol Reprod. 1995;53:664–669. - PubMed
-
- Peluso JJ. Placing progesterone in the apoptotic pathway. Trends Endocrinol Metab. 1997;8:267–271. - PubMed
-
- Peluso JJ, Pappalardo A. Progesterone mediates its anti-mitogenic and anti-apoptotic actions in rat granulosa cells through a progesterone-binding protein with gamma aminobutyric acidA receptor-like features. Biol Reprod. 1998;58:1131–1137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

