Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women
- PMID: 23242542
- PMCID: PMC3636781
- DOI: 10.1093/infdis/jis619
Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women
Abstract
Background: Cervical cancer is a major public health problem for women in sub-Saharan Africa. Availability of a human papillomavirus (HPV) vaccine could have an important public health impact.
Methods: In this phase IIIb, double-blind, randomized, placebo-controlled, multicenter trial (NCT00481767), healthy African girls and young women seronegative for human immunodeficiency virus (HIV) were stratified by age (10-14 or 15-25 years) and randomized (2:1) to receive either HPV-16/18 AS04-adjuvanted vaccine (n = 450) or placebo (n = 226) at 0, 1, and 6 months. The primary objective was to evaluate HPV-16/18 antibody responses at month 7. Seropositivity rates and corresponding geometric mean titers (GMTs) were measured by enzyme-linked immunosorbent assay.
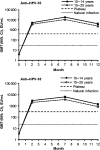
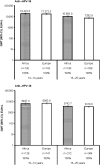
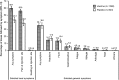
Results: In the according-to-protocol analysis at month 7, 100% of initially seronegative participants in the vaccine group were seropositive for both anti-HPV-16 and anti-HPV-18 antibodies (n = 130 and n = 128 for 10-14-year-olds, respectively; n = 190 and n = 212 for 15-25-year-olds). GMTs for HPV-16 and HPV-18 were higher in 10-14-year-olds (18 423 [95% confidence interval, 16 185-20 970] and 6487 [5590-7529] enzyme-linked immunosorbent assay units (EU)/mL, respectively) than in 15-25-year-olds (10 683 [9567-11 930] and 3743 [3400-4120] EU/mL, respectively). Seropositivity was maintained at month 12. No participant withdrew owing to adverse events. No vaccine-related serious adverse events were reported.
Conclusions: The HPV-16/18 AS04-adjuvanted vaccine was highly immunogenic and had a clinically acceptable safety profile when administered to healthy HIV-seronegative African girls and young women.
Figures

References
-
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. - PubMed
-
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Human papillomavirus and related cancers in Africa. Summary report 2010 www.who.int/hpvcentre. Accessed 27 January 2012.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

