Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth
- PMID: 23242557
- PMCID: PMC3730266
- DOI: 10.1002/mrd.22144
Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth
Abstract
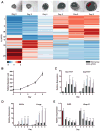
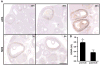
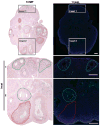
In vitro follicle growth has emerged as a technology that can provide new information about folliculogenesis and serve as part of a suite of methods currently under development to assist women whose fertility is threatened by cancer treatments. Though it has been shown that in vitro-grown follicles secrete peptide and steroid hormones, much of the follicular transcriptome remains unknown. Thus, microarray analysis was performed to characterize the transcriptome and secretome of in vitro-grown follicles. One prominently regulated gene product was cartilage oligomeric matrix protein (Comp): its mRNA was upregulated during the final 4 days of culture (P < 0.05) and COMP protein could be detected in medium from individual follicles. COMP expression localized to mural granulosa cells of large antral follicles both in vitro and in vivo, with maximal expression immediately preceding ovulation in cycling and chorionic gonadotropin-primed female mice. COMP was co-expressed with two known markers of follicle maturation, inhibin β(A) and gremlin, and was expressed only in TUNEL-negative follicles. In addition to other gene products identified in the microarray, COMP has potential utility as a marker of follicle maturation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



Similar articles
-
The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development.Hum Reprod. 2016 Apr;31(4):866-74. doi: 10.1093/humrep/dew013. Epub 2016 Feb 13. Hum Reprod. 2016. PMID: 26874357
-
Follicle-restricted compartmentalization of transforming growth factor beta superfamily ligands in the feline ovary.Biol Reprod. 2004 Mar;70(3):846-59. doi: 10.1095/biolreprod.103.021857. Epub 2003 Dec 3. Biol Reprod. 2004. PMID: 14656728
-
The activin-follistatin system and in vitro early follicle development in goats.J Endocrinol. 2006 Apr;189(1):113-25. doi: 10.1677/joe.1.06487. J Endocrinol. 2006. PMID: 16614386
-
Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage.Mol Endocrinol. 2006 Oct;20(10):2456-68. doi: 10.1210/me.2005-0357. Epub 2006 Jun 1. Mol Endocrinol. 2006. PMID: 16740654
-
Activin B is produced early in antral follicular development and suppresses thecal androgen production.Reproduction. 2012 May;143(5):637-50. doi: 10.1530/REP-11-0327. Epub 2012 Mar 26. Reproduction. 2012. PMID: 22450673 Free PMC article.
Cited by
-
An ex vivo ovulation system enables the discovery of novel ovulatory pathways and nonhormonal contraceptive candidates†.Biol Reprod. 2023 Apr 11;108(4):629-644. doi: 10.1093/biolre/ioad009. Biol Reprod. 2023. PMID: 36708230 Free PMC article.
-
Capitalizing on transcriptome profiling to optimize and identify targets for promoting early murine folliculogenesis in vitro.Sci Rep. 2021 Jun 15;11(1):12517. doi: 10.1038/s41598-021-92036-y. Sci Rep. 2021. PMID: 34131220 Free PMC article.
-
Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles.Biol Reprod. 2015 Jan;92(1):23. doi: 10.1095/biolreprod.114.121756. Epub 2014 Nov 5. Biol Reprod. 2015. PMID: 25376232 Free PMC article.
-
Supplemented αMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels.Biotechnol Bioeng. 2013 Dec;110(12):3258-68. doi: 10.1002/bit.24986. Epub 2013 Jul 15. Biotechnol Bioeng. 2013. PMID: 23801027 Free PMC article.
-
Recapitulating folliculogenesis and oogenesis outside the body: encapsulated in vitro follicle growth†.Biol Reprod. 2023 Jan 14;108(1):5-22. doi: 10.1093/biolre/ioac176. Biol Reprod. 2023. PMID: 36136744 Free PMC article.
References
-
- Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–688. - PubMed
-
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24hours. Hum Reprod. 1999;14:1299–1301. - PubMed
-
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–146. - PubMed
-
- Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: Clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–898. - PubMed
-
- Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

