Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas
- PMID: 23242601
- PMCID: PMC3659948
- DOI: 10.3324/haematol.2012.077917
Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas
Abstract
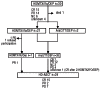
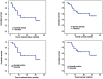
The prognosis of patients with central nervous system relapse of aggressive lymphoma is very poor with no therapy established so far. In a prospective multicenter phase II study, we evaluated a potentially curative chemotherapy-only regimen in these patients. Adult immunocompetent patients 65 years of age or under received induction chemotherapy with MTX/IFO/DEP (methotrexate 4 g/m(2) intravenously (i.v.) Day 1, ifosfamide 2 g/m(2) i.v. Days 3- 5 and liposomal cytarabine 50 mg intrathecally (i.th) Day 6) and AraC/TT/DEP (cytarabine 3g/m(2) i.v. Days 1-2, thiotepa 40 mg/m(2) i.v. Day 2 and i.th. liposomal cytarabine 50 mg i.th. Day 3) followed by high-dose chemotherapy with carmustine 400 mg/m(2) i.v. Day -5, thiotepa 2×5 mg/kg i.v. Days -4 to -3 and etoposide 150 mg/m(2) i.v. Days -5 to -3, and autologous stem cell transplantation Day 0 (HD-ASCT). Thirty eligible patients (median age 58 years) were enrolled. After HD-ASCT (n=24), there was a complete remission in 15 (63%), partial remission in 2 (8%) and progressive disease in 7 (29%) patients. Myelotoxicity was the most adverse event with CTC grade 3/4 infections in 12% of MTX/IFO/DEP courses, 21% of AraC/TT/DEP courses and 46% of HD-ASCT courses. The 2-year time to treatment failure was 49%±19 for all patients and 58%±22 for patients completing HD-ASCT. The protocol assessed proved feasible and highly active with long-lasting remissions in a large proportion of patients. (ClinicalTrials.govIdentifier NCT01148173).
Figures
References
-
- Bierman P, Giglio P. Diagnosis and treatment of central nervous system involvement in non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2005; 19(4):597–609 - PubMed
-
- Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL). Ann Oncol. 2007;18(1):149–57 - PubMed
-
- Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009; 113(17):3896–902 - PubMed
-
- Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol. 2004;15(1):129–33 - PubMed
-
- Schmitz N, Zeynalova S, Glass B, Kaiser U, Cavallin-Stahl E, Wolf M, et al. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann Oncol. 2012;23(5):1267–73 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

