Ontogeny of erythroid gene expression
- PMID: 23243273
- PMCID: PMC3567347
- DOI: 10.1182/blood-2012-04-422394
Ontogeny of erythroid gene expression
Abstract
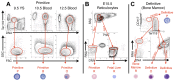
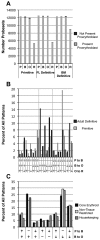
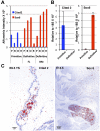
Erythroid ontogeny is characterized by overlapping waves of primitive and definitive erythroid lineages that share many morphologic features during terminal maturation but have marked differences in cell size and globin expression. In the present study, we compared global gene expression in primitive, fetal definitive, and adult definitive erythroid cells at morphologically equivalent stages of maturation purified from embryonic, fetal, and adult mice. Surprisingly, most transcriptional complexity in erythroid precursors is already present by the proerythroblast stage. Transcript levels are markedly modulated during terminal erythroid maturation, but housekeeping genes are not preferentially lost. Although primitive and definitive erythroid lineages share a large set of nonhousekeeping genes, annotation of lineage-restricted genes shows that alternate gene usage occurs within shared functional categories, as exemplified by the selective expression of aquaporins 3 and 8 in primitive erythroblasts and aquaporins 1 and 9 in adult definitive erythroblasts. Consistent with the known functions of Aqp3 and Aqp8 as H2O2 transporters, primitive, but not definitive, erythroblasts preferentially accumulate reactive oxygen species after exogenous H2O2 exposure. We have created a user-friendly Web site (http://www.cbil.upenn.edu/ErythronDB) to make these global expression data readily accessible and amenable to complex search strategies by the scientific community.
Figures




References
-
- Bloom W, Fawcett DW. A Textbook of Histology. 10th Ed. Philadelphia, PA: Saunders; 1975.
-
- Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11(3):87–93. - PubMed
-
- Maximow AA. Untersuchungen uber blut und bindegewebe 1. Die fruhesten entwicklungsstadien der blut- und binde- gewebszellan bein saugetierembryo, bis zum anfang der blutbilding unden leber. Arch Mikroskop Anat. 1909;73:444–561.
-
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. - PubMed
-
- McGrath KE, Koniski AD, Malik J, Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101(5):1669–1676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

