Retinoid agonist Am80-enhanced neutrophil bactericidal activity arising from granulopoiesis in vitro and in a neutropenic mouse model
- PMID: 23243275
- PMCID: PMC3567346
- DOI: 10.1182/blood-2012-06-436022
Retinoid agonist Am80-enhanced neutrophil bactericidal activity arising from granulopoiesis in vitro and in a neutropenic mouse model
Abstract
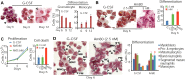
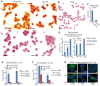
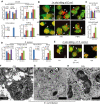
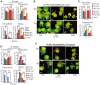
Despite advances in the therapeutic use of recombinant granulocyte colony-stimulating factor (G-CSF) to promote granulopoiesis of human hematopoietic stem cells (HSCs), neutropenia remains one of the most serious complications of cancer chemotherapy. We discovered that retinoid agonist Am80 (tamibarotene) is more potent than G-CSF in coordinating neutrophil differentiation and immunity development. Am80-induced neutrophils (AINs) either in vitro or in neutropenic mouse model displayed strong bactericidal activities, similar to those of human peripheral blood neutrophils (PBNs) or mouse peripheral blood neutrophils (MPBNs) but markedly greater than did G-CSF–induced neutrophils (GINs). In contrast to GINs but similar to PBNs, the enhanced bacterial killing by AINs accompanied both better granule maturation and greater coexpression of CD66 antigen with the integrin β2 subunit CD18. Consistently, anti-CD18 antibody neutralized Am80-induced bactericidal activities of AINs. These studies demonstrate that Am80 is more effective than G-CSF in promoting neutrophil differentiation and bactericidal activities, probably through coordinating the functional interaction of CD66 with CD18 to enhance the development of neutrophil immunity during granulopoiesis. Our findings herein suggest a molecular rationale for developing new therapy against neutropenia using Am80 as a cost-effective treatment option.
Figures


Similar articles
-
Strategies to generate functionally normal neutrophils to reduce infection and infection-related mortality in cancer chemotherapy.Pharmacol Ther. 2019 Dec;204:107403. doi: 10.1016/j.pharmthera.2019.107403. Epub 2019 Aug 27. Pharmacol Ther. 2019. PMID: 31470030 Free PMC article. Review.
-
Am80-GCSF synergizes myeloid expansion and differentiation to generate functional neutrophils that reduce neutropenia-associated infection and mortality.EMBO Mol Med. 2016 Nov 2;8(11):1340-1359. doi: 10.15252/emmm.201606434. Print 2016 Nov. EMBO Mol Med. 2016. PMID: 27737899 Free PMC article.
-
Am80, a retinoic acid receptor agonist, ameliorates murine vasculitis through the suppression of neutrophil migration and activation.Arthritis Rheum. 2013 Feb;65(2):503-12. doi: 10.1002/art.37784. Arthritis Rheum. 2013. PMID: 23203767
-
A specific stimulator of granulocyte colony-stimulating factor accelerates recovery from cyclophosphamide-induced neutropenia in the mouse.Blood. 1997 Jul 15;90(2):795-802. Blood. 1997. PMID: 9226180
-
Tamibarotene: a candidate retinoid drug for Alzheimer's disease.Biol Pharm Bull. 2012;35(8):1206-12. doi: 10.1248/bpb.b12-00314. Biol Pharm Bull. 2012. PMID: 22863914 Review.
Cited by
-
Strategies to generate functionally normal neutrophils to reduce infection and infection-related mortality in cancer chemotherapy.Pharmacol Ther. 2019 Dec;204:107403. doi: 10.1016/j.pharmthera.2019.107403. Epub 2019 Aug 27. Pharmacol Ther. 2019. PMID: 31470030 Free PMC article. Review.
-
Permissive lung neutrophils facilitate tuberculosis immunopathogenesis in male phagocyte NADPH oxidase-deficient mice.PLoS Pathog. 2024 Aug 23;20(8):e1012500. doi: 10.1371/journal.ppat.1012500. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39178329 Free PMC article.
-
Selection of the Inducer for the Differentiation of Chicken Embryonic Stem Cells into Male Germ Cells In Vitro.PLoS One. 2016 Oct 14;11(10):e0164664. doi: 10.1371/journal.pone.0164664. eCollection 2016. PLoS One. 2016. PMID: 27741318 Free PMC article.
-
Am80-GCSF synergizes myeloid expansion and differentiation to generate functional neutrophils that reduce neutropenia-associated infection and mortality.EMBO Mol Med. 2016 Nov 2;8(11):1340-1359. doi: 10.15252/emmm.201606434. Print 2016 Nov. EMBO Mol Med. 2016. PMID: 27737899 Free PMC article.
-
Concise review: next-generation cell therapies to prevent infections in neutropenic patients.Stem Cells Transl Med. 2014 Apr;3(4):541-8. doi: 10.5966/sctm.2013-0145. Epub 2014 Mar 5. Stem Cells Transl Med. 2014. PMID: 24598780 Free PMC article. Review.
References
-
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. - PubMed
-
- Hartmann LC, Tschetter LK, Habermann TM, et al. Granulocyte colony-stimulating factor in severe chemotherapy-induced afebrile neutropenia. N Engl J Med. 1997;336(25):1776–1780. - PubMed
-
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. - PubMed
-
- Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147(6):400–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

