HIV Vaccine Trials Network: activities and achievements of the first decade and beyond
- PMID: 23243491
- PMCID: PMC3521567
- DOI: 10.4155/cli.12.8
HIV Vaccine Trials Network: activities and achievements of the first decade and beyond
Abstract
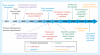
The HIV Vaccine Trials Network (HVTN) is an international collaboration of scientists and educators facilitating the development of HIV/AIDS preventive vaccines. The HVTN conducts all phases of clinical trials, from evaluating experimental vaccines for safety and immunogenicity, to testing vaccine efficacy. Over the past decade, the HVTN has aimed to improve the process of designing, implementing and analyzing vaccine trials. Several major achievements include streamlining protocol development while maintaining input from diverse stakeholders, establishing a laboratory program with standardized assays and systems allowing for reliable immunogenicity assessments across trials, setting statistical standards for the field and actively engaging with site communities. These achievements have allowed the HVTN to conduct over 50 clinical trials and make numerous scientific contributions to the field.
Figures

References
-
- Goepfert PA, Tomaras GD, Horton H, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25(3):510–518. - PubMed
-
- Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 2008;82(24):12449–12463. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
