Quantitative OCT angiography of optic nerve head blood flow
- PMID: 23243564
- PMCID: PMC3521313
- DOI: 10.1364/BOE.3.003127
Quantitative OCT angiography of optic nerve head blood flow
Abstract
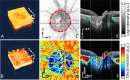
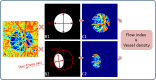
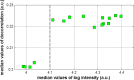
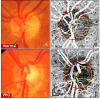
Optic nerve head (ONH) blood flow may be associated with glaucoma development. A reliable method to quantify ONH blood flow could provide insight into the vascular component of glaucoma pathophysiology. Using ultrahigh-speed optical coherence tomography (OCT), we developed a new 3D angiography algorithm called split-spectrum amplitude-decorrelation angiography (SSADA) for imaging ONH microcirculation. In this study, a method to quantify SSADA results was developed and used to detect ONH perfusion changes in early glaucoma. En face maximum projection was used to obtain 2D disc angiograms, from which the average decorrelation values (flow index) and the percentage area occupied by vessels (vessel density) were computed from the optic disc and a selected region within it. Preperimetric glaucoma patients had significant reductions of ONH perfusion compared to normals. This pilot study indicates OCT angiography can detect the abnormalities of ONH perfusion and has the potential to reveal the ONH blood flow mechanism related to glaucoma.
Keywords: (170.3880) Medical and biological imaging; (170.4470) Ophthalmology; (170.4500) Optical coherence tomography.
Figures

References
-
- Quigley H. A., Vitale S., “Models of open-angle glaucoma prevalence and incidence in the United States,” Invest. Ophthalmol. Vis. Sci. 38(1), 83–91 (1997). - PubMed
-
- Hayreh S. S., “Progress in the understanding of the vascular etiology of glaucoma,” Curr. Opin. Ophthalmol. 5(2), 26–35 (1994).10.1097/00055735-199404000-00006 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
