Rh(III)-catalyzed regioselective synthesis of pyridines from alkenes and α,β-unsaturated oxime esters
- PMID: 23244023
- PMCID: PMC3541442
- DOI: 10.1021/ja3104389
Rh(III)-catalyzed regioselective synthesis of pyridines from alkenes and α,β-unsaturated oxime esters
Abstract
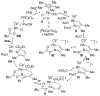
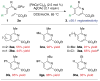
α,β-Unsaturated O-pivaloyl oximes are coupled to alkenes by Rh(III) catalysis to afford substituted pyridines. The reaction with activated alkenes is exceptionally regioselective and high-yielding. Mechanistic studies suggest that heterocycle formation proceeds via reversible C-H activation, alkene insertion, and a C-N bond formation/N-O bond cleavage process.
Conflict of interest statement
Funding Sources
No competing financial interests have been declared.
Figures


Similar articles
-
Rh(III)-catalyzed decarboxylative coupling of acrylic acids with unsaturated oxime esters: carboxylic acids serve as traceless activators.J Am Chem Soc. 2014 Feb 19;136(7):2735-8. doi: 10.1021/ja412444d. Epub 2014 Feb 10. J Am Chem Soc. 2014. PMID: 24512241 Free PMC article.
-
Expedient Access to 2,3-Dihydropyridines from Unsaturated Oximes by Rh(III)-Catalyzed C-H Activation.J Am Chem Soc. 2015 Jul 22;137(28):8892-5. doi: 10.1021/jacs.5b04946. Epub 2015 Jul 8. J Am Chem Soc. 2015. PMID: 26154248 Free PMC article.
-
Regioselective synthesis of 1,2-dihydropyridines by rhodium-catalyzed hydroboration of pyridines.J Am Chem Soc. 2012 Feb 29;134(8):3699-702. doi: 10.1021/ja3002953. Epub 2012 Feb 17. J Am Chem Soc. 2012. PMID: 22320932
-
Oxime Esters: Flexible Building Blocks for Heterocycle Formation.Top Curr Chem (Cham). 2023 May 18;381(4):17. doi: 10.1007/s41061-023-00431-y. Top Curr Chem (Cham). 2023. PMID: 37202650 Review.
-
Recent Advances in N-O Bond Cleavage of Oximes and Hydroxylamines to Construct N-Heterocycle.Molecules. 2023 Feb 13;28(4):1775. doi: 10.3390/molecules28041775. Molecules. 2023. PMID: 36838760 Free PMC article. Review.
Cited by
-
Facile Rh(III)-Catalyzed Synthesis of Fluorinated Pyridines.Org Lett. 2015 Jun 5;17(11):2567-9. doi: 10.1021/acs.orglett.5b00979. Epub 2015 May 20. Org Lett. 2015. PMID: 25992591 Free PMC article.
-
Rhodium-catalyzed C-H functionalization-based approach to eight-membered lactams.Chem Sci. 2015 Apr 1;6(4):2275-2285. doi: 10.1039/c5sc00092k. Epub 2015 Feb 5. Chem Sci. 2015. PMID: 29308141 Free PMC article.
-
Isothiourea-mediated one-pot synthesis of functionalized pyridines.Angew Chem Int Ed Engl. 2013 Oct 25;52(44):11642-6. doi: 10.1002/anie.201306786. Epub 2013 Sep 17. Angew Chem Int Ed Engl. 2013. PMID: 24106063 Free PMC article.
-
Rh(III)-catalyzed decarboxylative coupling of acrylic acids with unsaturated oxime esters: carboxylic acids serve as traceless activators.J Am Chem Soc. 2014 Feb 19;136(7):2735-8. doi: 10.1021/ja412444d. Epub 2014 Feb 10. J Am Chem Soc. 2014. PMID: 24512241 Free PMC article.
-
Rhodium(III)-catalyzed dearomatizing (3 + 2) annulation of 2-alkenylphenols and alkynes.J Am Chem Soc. 2014 May 28;136(21):7607-10. doi: 10.1021/ja5034952. Epub 2014 May 19. J Am Chem Soc. 2014. PMID: 24820195 Free PMC article.
References
-
-
For reviews, see: Hill MD. Chem Eur J. 2010;16:12052.Henry GD. Tetrahedron. 2004;60:6043.
-
-
-
For reviews of pyridine syntheses by carbonyl condensation, see: Bagley MC, Glover C, Merritt EA. Synlett. 2007:2459.Karchenko VG, Markova LI, Fedotova OV, Pchelintseva NV. Chem Heterocyc Compd. 2003;39:1121–1141.Sausins A, Duburs G. Heterocycles. 1988;27:269.
-
-
-
For reviews of pyridine syntheses by [2+2+2] cycloadditions of alkynes and nitriles, see: Heller B, Hapke M. Chem Soc Rev. 2007;36:1085.Varela JA, Saá C. Chem Rev. 2003;103:3787.Selected examples: Chang HT, Jeganmohan M, Cheng CH. Org Lett. 2007;9:505.McCormick MM, Duong HA, Zuo G, Louie J. J Am Chem Soc. 2005;127:5030.Tanaka R, Yuza A, Suzuki D, Takayama Y, Sato F, Urabe H. J Am Chem Soc. 2005;127:7774.
-
-
-
For leading examples, see: Gati W, Rammah MM, Rammah MB, Couty F, Evano G. J Am Chem Soc. 2012;134:9078.Chen MZ, Micalizio GC. J Am Chem Soc. 2012;134:1352.Ohashi M, Takeda I, Ikawa M, Ogoshi S. J Am Chem Soc. 2011;133:18018.Wang YF, Toh KK, Ng EPJ, Chiba S. J Am Chem Soc. 2011;133:6411.Wang YF, Chiba S. J Am Chem Soc. 2009;131:12570.Manning JR, Davies HML. J Am Chem Soc. 2008;130:8602.Liu S, Liebeskind LS. J Am Chem Soc. 2008;130:6918.Movassaghi M, Hill MD, Ahmad OK. J Am Chem Soc. 2007;129:10096.Movassaghi M, Hill MD. J Am Chem Soc. 2006;128:4592.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

