BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model
- PMID: 23244588
- PMCID: PMC4361002
- DOI: 10.3727/096368912X658854
BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model
Abstract
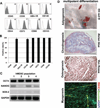
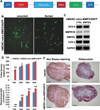
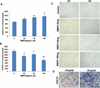
Muscle-derived cells have been successfully isolated using a variety of different methods and have been shown to possess multilineage differentiation capacities, including an ability to differentiate into articular cartilage and bone in vivo; however, the characterization of human muscle-derived stem cells (hMDSCs) and their bone regenerative capacities have not been fully investigated. Genetic modification of these cells may enhance their osteogenic capacity, which could potentially be applied to bone regenerative therapies. We found that hMDSCs, isolated by the preplate technique, consistently expressed the myogenic marker CD56, the pericyte/endothelial cell marker CD146, and the mesenchymal stem cell markers CD73, CD90, CD105, and CD44 but did not express the hematopoietic stem cell marker CD45, and they could undergo osteogenic, chondrogenic, adipogenic, and myogenic differentiation in vitro. In order to investigate the osteoinductive potential of hMDSCs, we constructed a retroviral vector expressing BMP4 and GFP and a lentiviral vector expressing BMP2. The BMP4-expressing hMDSCs were able to undergo osteogenic differentiation in vitro and exhibited enhanced mineralization compared to nontransduced cells; however, when transplanted into a calvarial defect, they failed to regenerate bone. Local administration of BMP4 protein and cell pretreatment with N-acetylcysteine (NAC), which improves cell survival, did not enhance the osteogenic capacity of the retro-BMP4-transduced cells. In contrast, lenti-BMP2-transduced hMDSCs not only exhibited enhanced in vitro osteogenic differentiation but also induced robust bone formation and nearly completely healed a critical-sized calvarial defect in CD-1 nude mice 6 weeks following transplantation. Herovici's staining of the regenerated bone demonstrated that the bone matrix contained a large amount of type I collagen. Our findings indicated that the hMDSCs are likely mesenchymal stem cells of muscle origin and that BMP2 is more efficient than BMP4 in promoting the bone regenerative capacity of the hMDSCs in vivo.
Conflict of interest statement
All the other authors have no potential conflict of interest to disclose.
Figures




Similar articles
-
Influences of donor and host age on human muscle-derived stem cell-mediated bone regeneration.Stem Cell Res Ther. 2018 Nov 21;9(1):316. doi: 10.1186/s13287-018-1066-z. Stem Cell Res Ther. 2018. PMID: 30463597 Free PMC article.
-
A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP.Biomaterials. 2014 Aug;35(25):6859-70. doi: 10.1016/j.biomaterials.2014.04.113. Epub 2014 May 21. Biomaterials. 2014. PMID: 24856105 Free PMC article.
-
Characterization of the chondrogenic and osteogenic potential of male and female human muscle-derived stem cells: Implication for stem cell therapy.J Orthop Res. 2019 Jun;37(6):1339-1349. doi: 10.1002/jor.24231. Epub 2019 Feb 21. J Orthop Res. 2019. PMID: 30667562
-
Concise review: bridging the gap: bone regeneration using skeletal stem cell-based strategies - where are we now?Stem Cells. 2014 Jan;32(1):35-44. doi: 10.1002/stem.1559. Stem Cells. 2014. PMID: 24115290 Review.
-
* Calvarial Defects: Cell-Based Reconstructive Strategies in the Murine Model.Tissue Eng Part C Methods. 2017 Dec;23(12):971-981. doi: 10.1089/ten.TEC.2017.0230. Epub 2017 Oct 4. Tissue Eng Part C Methods. 2017. PMID: 28825366 Free PMC article. Review.
Cited by
-
Herpes simplex viral-vector design for efficient transduction of nonneuronal cells without cytotoxicity.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):E1632-41. doi: 10.1073/pnas.1423556112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775541 Free PMC article.
-
Hypoxia-inducible factor 1α (HIF-1α) is a major determinant in the enhanced function of muscle-derived progenitors from MRL/MpJ mice.FASEB J. 2019 Jul;33(7):8321-8334. doi: 10.1096/fj.201801794R. Epub 2019 Apr 10. FASEB J. 2019. PMID: 30970214 Free PMC article.
-
Role of donor and host cells in muscle-derived stem cell-mediated bone repair: differentiation vs. paracrine effects.FASEB J. 2014 Aug;28(8):3792-809. doi: 10.1096/fj.13-247965. Epub 2014 May 19. FASEB J. 2014. PMID: 24843069 Free PMC article.
-
Influences of donor and host age on human muscle-derived stem cell-mediated bone regeneration.Stem Cell Res Ther. 2018 Nov 21;9(1):316. doi: 10.1186/s13287-018-1066-z. Stem Cell Res Ther. 2018. PMID: 30463597 Free PMC article.
-
Recent Advances and Future of Gene Therapy for Bone Regeneration.Curr Osteoporos Rep. 2018 Aug;16(4):504-511. doi: 10.1007/s11914-018-0459-3. Curr Osteoporos Rep. 2018. PMID: 29909597 Review.
References
-
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone. Miner. Res. 2010;25(7):1468–1486. - PubMed
-
- Chou YF, Zuk PA, Chang TL, Benhaim P, Wu BM. Adipose-derived stem cells and BMP2: Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect. Tissue Res. 2011;52(2):109–118. - PubMed
-
- Chuang CK, Lin KJ, Lin CY, Chang YH, Yen TC, Hwang SM, Sung LY, Chen HC, Hu YC. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng. Part A. 2010;16(2):479–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

