Platelet factor 4 activity against P. falciparum and its translation to nonpeptidic mimics as antimalarials
- PMID: 23245326
- PMCID: PMC3638032
- DOI: 10.1016/j.chom.2012.10.017
Platelet factor 4 activity against P. falciparum and its translation to nonpeptidic mimics as antimalarials
Abstract
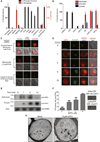
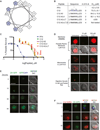
Plasmodium falciparum pathogenesis is affected by various cell types in the blood, including platelets, which can kill intraerythrocytic malaria parasites. Platelets could mediate these antimalarial effects through human defense peptides (HDPs), which exert antimicrobial effects by permeabilizing membranes. Therefore, we screened a panel of HDPs and determined that human platelet factor 4 (hPF4) kills malaria parasites inside erythrocytes by selectively lysing the parasite digestive vacuole (DV). PF4 rapidly accumulates only within infected erythrocytes and is required for parasite killing in infected erythrocyte-platelet cocultures. To exploit this antimalarial mechanism, we tested a library of small, nonpeptidic mimics of HDPs (smHDPs) and identified compounds that kill P. falciparum by rapidly lysing the parasite DV while sparing the erythrocyte plasma membrane. Lead smHDPs also reduced parasitemia in a murine malaria model. Thus, identifying host molecules that control parasite growth can further the development of related molecules with therapeutic potential.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Current Opinion in Colloid & Interface Science. 2009;14:349–355.
-
- Eslin DE, Zhang C, Samuels KJ, Rauova L, Zhai L, Niewiarowski S, Cines DB, Poncz M, Kowalska MA. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–3180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

