Efficacy of Pegylated interferon α-2a and α-2b in patients with genotype 1 chronic hepatitis C: a meta-analysis
- PMID: 23245594
- PMCID: PMC3556138
- DOI: 10.1186/1471-2334-12-357
Efficacy of Pegylated interferon α-2a and α-2b in patients with genotype 1 chronic hepatitis C: a meta-analysis
Abstract
Background: Two formulations of Pegylated interferon (Peg-IFN) are on the market for treatment of chronic hepatitis C virus (HCV) infection. The purpose of this meta-analysis was to assess the efficacy of Peg-IFN α-2a versus Peg-IFN α-2b in combination with ribavirin in anti-human immunodeficiency virus (HIV)-negative patients with genotype 1 chronic HCV infection.
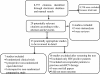
Methods: The following criteria were to be met for inclusion in the meta-analysis: (a) original data from randomized and non-randomized clinical trials; (b) study on the efficacy of conventional doses of Peg-IFN α-2a (180 μg/week) versus Peg-IFN α-2b (1.5 μg/kg of body weight/week), both in combination with ribavirin, in antiviral therapy-naïve HCV-genotype 1 subjects; (c) at least one of these primary outcomes: Rapid Virological Response (RVR); Early Complete Virological Response (EVR); End of Treatment Response (ETR); Sustained Virological Response (SVR); (d) odds ratio estimates of relative risk (RR) and associated 95% confidence intervals (CIs) or at least data enabling them to be computed; (e) English language; and (f) published as a full paper up to December 2011.
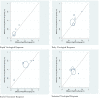
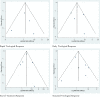
Results: Seven published studies met the inclusion criteria, allowing a meta-analysis on 3,026 patients. Peg-IFN α-2a and Peg-IFN α-2b showed similar rate of RVR (RR = 1.05; 95% CI = 0.87-1.27, p = 0.62) and SVR (RR = 1.08; 95% CI = 0.99-1.18, p = 0.098). Peg-IFN α-2a more frequently than Peg-IFN α-2b achieved EVR (RR = 1.11; 95% CI = 1.02-1.21, p = 0.013) and ETR (RR = 1.22; 95% CI = 1.14-1.31, p < 0.0001).
Conclusion: The standard schedules of Peg-IFN α-2a and Peg-IFN α-2b, both in combination with ribavirin, can be used indifferently for patients with chronic HCV genotype 1 who are anti- to eliminate HIV-negative and antiviral treatment-naïve.
Figures
References
-
- Practice guidelines for the treatment of hepatitis C. Practice guidelines for the treatment of hepatitis C: Recommendations from an AISF/SIMIT/SIMAST Expert Opinion Meeting. Dig Liv Dis. 2010;42:81–91. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

