Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people
- PMID: 23245760
- PMCID: PMC3898083
- DOI: 10.1016/j.biopsych.2012.10.022
Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people
Abstract
Background: Binge eating is associated with obesity and has been conceptualized as "food addiction." However, this view has received only inconsistent support in humans, and limited evidence relates key neurocircuitry to the disorder. Moreover, relatively few studies have used pharmacologic functional magnetic resonance imaging to probe the underlying basis of altered eating behaviors.
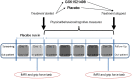
Methods: In a double-blind, placebo-controlled, parallel group study, we explored the effects of a potent mu-opioid receptor antagonist, GSK1521498, in obese individuals with moderate binge eating. Subjects were tested during a baseline placebo run-in period and retested after 28-days of drug (n = 21) or placebo (n = 21) treatment. Using functional magnetic resonance imaging and behavioral measures, we determined the drug's effects on brain responses to food images and, separately, on motivation to expend energy to view comparable images.
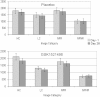
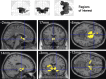
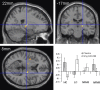
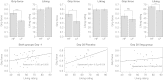
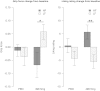
Results: Compared with placebo, GSK1521498 was associated with a significant reduction in pallidum/putamen responses to pictures of high-calorie food and a reduction in motivation to view images of high-calorie food. Intriguingly, although motivational responding was reduced, subjective liking for the same images actually increased following drug treatment.
Conclusions: Stimulus-specific putamen/pallidal responses in obese people with binge eating are sensitive to altered mu-opioid function. This neuromodulation was accompanied by reductions in motivational responding, as measured by grip force, although subjective liking responses to the same stimuli actually increased. As well as providing evidence for a link between the opioid system and food-related behavior in binge-eating obese individuals, these results support a dissociation across measures of motivation and liking associated with food-related stimuli in these individuals.
Trial registration: ClinicalTrials.gov NCT01195792.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Berridge K.C. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
-
- Robbins T.W., Everitt B.J. A role for mesencephalic dopamine in activation: Commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191:433–437. - PubMed
-
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

