Functional insights from the crystal structure of the N-terminal domain of the prototypical toll receptor
- PMID: 23245851
- PMCID: PMC3542428
- DOI: 10.1016/j.str.2012.11.003
Functional insights from the crystal structure of the N-terminal domain of the prototypical toll receptor
Abstract
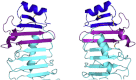
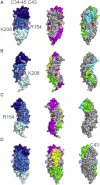
Drosophila melanogaster Toll is the founding member of an important family of pathogen-recognition receptors in humans, the Toll-like receptor (TLR) family. In contrast, the prototypical receptor is a cytokine-like receptor for Spätzle (Spz) protein and plays a dual role in both development and immunity. Here, we present the crystal structure of the N-terminal domain of the receptor that encompasses the first 201 amino acids at 2.4 Å resolution. To our knowledge, the cysteine-rich cap adopts a novel fold unique to Toll-1 orthologs in insects and that is not critical for ligand binding. However, we observed that an antibody directed against the first ten LRRs blocks Spz signaling in a Drosophila cell-based assay. Supplemented by point mutagenesis and deletion analysis, our data suggests that the region up to LRR 14 is involved in Spz binding. Comparison with mammalian TLRs reconciles previous contradictory findings about the mechanism of Toll activation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Cytokine Spatzle binds to the Drosophila immunoreceptor Toll with a neurotrophin-like specificity and couples receptor activation.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20461-6. doi: 10.1073/pnas.1317002110. Epub 2013 Nov 26. Proc Natl Acad Sci U S A. 2013. PMID: 24282309 Free PMC article.
-
Structure of the Toll-Spatzle complex, a molecular hub in Drosophila development and innate immunity.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6281-6. doi: 10.1073/pnas.1320678111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733933 Free PMC article.
-
Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spätzle.J Biol Chem. 2008 May 23;283(21):14629-35. doi: 10.1074/jbc.M800112200. Epub 2008 Mar 17. J Biol Chem. 2008. PMID: 18347020
-
The Drosophila Toll signaling pathway.J Immunol. 2011 Jan 15;186(2):649-56. doi: 10.4049/jimmunol.1002302. J Immunol. 2011. PMID: 21209287 Review.
-
The structural biology of Toll-like receptors.Structure. 2011 Apr 13;19(4):447-59. doi: 10.1016/j.str.2011.02.004. Structure. 2011. PMID: 21481769 Free PMC article. Review.
Cited by
-
GABAAR α2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance.Psychopharmacology (Berl). 2019 Oct;236(10):3023-3043. doi: 10.1007/s00213-019-05220-4. Epub 2019 Apr 27. Psychopharmacology (Berl). 2019. PMID: 31030249 Review.
-
Structure of the OsSERK2 leucine-rich repeat extracellular domain.Acta Crystallogr D Biol Crystallogr. 2014 Nov;70(Pt 11):3080-6. doi: 10.1107/S1399004714021178. Epub 2014 Oct 29. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 25372696 Free PMC article.
-
Liesegang-like patterns of Toll crystals grown in gel.J Appl Crystallogr. 2013 Apr 1;46(Pt 2):337-345. doi: 10.1107/S0021889812051606. Epub 2013 Feb 14. J Appl Crystallogr. 2013. PMID: 23596340 Free PMC article.
-
TLR Specific Immune Responses against Helminth Infections.J Parasitol Res. 2017;2017:6865789. doi: 10.1155/2017/6865789. Epub 2017 Oct 31. J Parasitol Res. 2017. PMID: 29225962 Free PMC article. Review.
-
Conventional and non-conventional Drosophila Toll signaling.Dev Comp Immunol. 2014 Jan;42(1):16-24. doi: 10.1016/j.dci.2013.04.011. Epub 2013 Apr 28. Dev Comp Immunol. 2014. PMID: 23632253 Free PMC article. Review.
References
-
- Anderson K.V., Bokla L., Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. - PubMed
-
- Aurikko J.P., Ruotolo B.T., Grossmann J.G., Moncrieffe M.C., Stephens E., Leppänen V.M., Robinson C.V., Saarma M., Bradshaw R.A., Blundell T.L. Characterization of symmetric complexes of nerve growth factor and the ectodomain of the pan-neurotrophin receptor, p75NTR. J. Biol. Chem. 2005;280:33453–33460. - PMC - PubMed
-
- Beck T., Krasauskas A., Gruene T., Sheldrick G.M. A magic triangle for experimental phasing of macromolecules. Acta Crystallogr. D Biol. Crystallogr. 2008;64:1179–1182. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

