A role for dopamine-mediated learning in the pathophysiology and treatment of Parkinson's disease
- PMID: 23246005
- PMCID: PMC3538862
- DOI: 10.1016/j.celrep.2012.11.014
A role for dopamine-mediated learning in the pathophysiology and treatment of Parkinson's disease
Erratum in
- Cell Rep. 2014 Jul 24;8(2):646. Bernandez, Maria Sol [corrected to Bernardez Sarria, Maria Sol]
Abstract
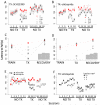
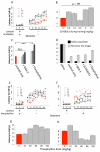
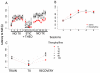
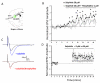
Dopamine contributes to corticostriatal plasticity and motor learning. Dopamine denervation profoundly alters motor performance, as in Parkinson's disease (PD); however, the extent to which these symptoms reflect impaired motor learning is unknown. Here, we demonstrate a D2 receptor blockade-induced aberrant learning that impedes future motor performance when dopamine signaling is restored, an effect diminished by coadministration of adenosine antagonists during blockade. We hypothesize that an inappropriate corticostriatal potentiation in striatopallidal cells of the indirect pathway underlies aberrant learning. We demonstrate synaptic potentiation in striatopallidal neurons induced by D2 blockade and diminished by application of an adenosine antagonist, consistent with behavioral observations. A neurocomputational model of the basal ganglia recapitulates the behavioral pattern and further links aberrant learning to plasticity in the indirect pathway. Thus, D2-mediated aberrant learning may contribute to motor deficits in PD, suggesting new avenues for the development of therapeutics.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Chronic Nicotine Mitigates Aberrant Inhibitory Motor Learning Induced by Motor Experience under Dopamine Deficiency.J Neurosci. 2016 May 11;36(19):5228-40. doi: 10.1523/JNEUROSCI.2754-15.2016. J Neurosci. 2016. PMID: 27170121 Free PMC article.
-
Aberrant plasticity and "learned" motor inhibition in Parkinson's disease.Sheng Li Xue Bao. 2012 Oct 25;64(5):543-9. Sheng Li Xue Bao. 2012. PMID: 23090495
-
Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models.Nature. 2007 Feb 8;445(7128):643-7. doi: 10.1038/nature05506. Nature. 2007. PMID: 17287809
-
The Enemy within: Propagation of Aberrant Corticostriatal Learning to Cortical Function in Parkinson's Disease.Front Neurol. 2013 Sep 12;4:134. doi: 10.3389/fneur.2013.00134. eCollection 2013. Front Neurol. 2013. PMID: 24062721 Free PMC article. Review.
-
Preservation of function in Parkinson's disease: what's learning got to do with it?Brain Res. 2011 Nov 14;1423:96-113. doi: 10.1016/j.brainres.2011.09.040. Epub 2011 Sep 29. Brain Res. 2011. PMID: 22000081 Free PMC article. Review.
Cited by
-
On the normative advantages of dopamine and striatal opponency for learning and choice.Elife. 2023 Mar 22;12:e85107. doi: 10.7554/eLife.85107. Elife. 2023. PMID: 36946371 Free PMC article.
-
Dopamine Selectively Modulates the Outcome of Learning Unnatural Action-Valence Associations.J Cogn Neurosci. 2017 May;29(5):816-826. doi: 10.1162/jocn_a_01099. Epub 2017 Jan 27. J Cogn Neurosci. 2017. PMID: 28129053 Free PMC article.
-
An integrative framework for perceptual disturbances in psychosis.Nat Rev Neurosci. 2019 Dec;20(12):763-778. doi: 10.1038/s41583-019-0234-1. Nat Rev Neurosci. 2019. PMID: 31712782 Review.
-
Engaging cognitive circuits to promote motor recovery in degenerative disorders. exercise as a learning modality.J Hum Kinet. 2016 Sep 10;52:35-51. doi: 10.1515/hukin-2015-0192. eCollection 2016 Sep 1. J Hum Kinet. 2016. PMID: 28149392 Free PMC article.
-
VTA projections to M1 are essential for reorganization of layer 2-3 network dynamics underlying motor learning.Nat Commun. 2025 Jan 2;16(1):200. doi: 10.1038/s41467-024-55317-4. Nat Commun. 2025. PMID: 39746993 Free PMC article.
References
-
- Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45:209–214. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17:587–592. - PubMed
-
- Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

