Pro- and antiarrhythmic effects of ATP-sensitive potassium current activation on reentry during early afterdepolarization-mediated arrhythmias
- PMID: 23246594
- PMCID: PMC4285341
- DOI: 10.1016/j.hrthm.2012.12.017
Pro- and antiarrhythmic effects of ATP-sensitive potassium current activation on reentry during early afterdepolarization-mediated arrhythmias
Abstract
Background: Under conditions promoting early afterdepolarizations (EADs), ventricular tissue can become bi-excitable, that is, capable of wave propagation mediated by either the Na current (INa) or the L-type calcium current (ICa,L), raising the possibility that ICa,L-mediated reentry may contribute to polymorphic ventricular tachycardia (PVT) and torsades de pointes. ATP-sensitive K current (IKATP) activation suppresses EADs, but the effects on ICa,L-mediated reentry are unknown.
Objective: To investigate the effects of IKATP activation on ICa,L-mediated reentry.
Methods: We performed optical voltage mapping in cultured neonatal rat ventricular myocyte monolayers exposed to BayK8644 and isoproterenol. The effects of pharmacologically activating IKATP with pinacidil were analyzed.
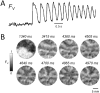
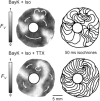
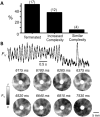
Results: In 13 monolayers with anatomic ICa,L-mediated reentry around a central obstacle, pinacidil (50 μM) converted ICa,L-mediated reentry to INa-mediated reentry. In 33 monolayers with functional ICa,L-mediated reentry (spiral waves), pinacidil terminated reentry in 17, converted reentry into more complex INa-mediated reentry resembling fibrillation in 12, and had no effect in 4. In simulated 2-dimensional bi-excitable tissue in which ICa,L- and INa-mediated wave fronts coexisted, slow IKATP activation (over minutes) reliably terminated rotors but rapid IKATP activation (over seconds) often converted ICa,L-mediated reentry to INa-mediated reentry resembling fibrillation.
Conclusions: IKATP activation can have proarrhythmic effects on EAD-mediated arrhythmias if ICa,L-mediated reentry is present.
Published by Elsevier Inc.
Figures
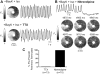
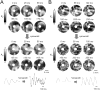

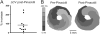

Comment in
-
Whirling dervishes from Pinacidil to pinwheels.Heart Rhythm. 2013 Apr;10(4):583-4. doi: 10.1016/j.hrthm.2013.01.009. Epub 2013 Jan 11. Heart Rhythm. 2013. PMID: 23313872 No abstract available.
References
-
- Fish FA, Prakash C, Roden DM. Suppression of repolarization-related arrhythmias in vitro and in vivo by low-dose potassium channel activators. Circ Res. 1990;82:1362–1369. - PubMed
-
- Spinelli W, Sorota S, Siegal M, et al. Antiarrhythmic actions of the ATP-regulated K current activated by pinacidil. Circ Res. 1991;68:1127–1137. - PubMed
-
- Carlsson L, Abrahamsson C, Drews L, et al. Antiarrhythmic effects of potassium channel openers in rhythm abnormalities related to delayed repolarization. Circulation. 1992;85:1491–1500. - PubMed
-
- Sato T, Hata Y, Yamamoto M, Morita H, et al. Early afterdepolarization abolished by potassium channel opener in a patient with idiopathic long QT syndrome. J Cardiovasc Electrophysiol. 1995;6:279–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

