Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy
- PMID: 23246599
- PMCID: PMC3605194
- DOI: 10.1016/j.hrthm.2012.12.016
Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy
Abstract
Background: Ventricular tachycardia (VT) is the second most common cause of death in patients with Duchenne muscular dystrophy (DMD). Recent studies have implicated enhanced sarcoplasmic reticulum (SR) Ca(2+) leak via type 2 ryanodine receptor (RyR2) as a cause of VT in the mdx mouse model of DMD. However, the signaling mechanisms underlying induction of SR Ca(2+) leak and VT are poorly understood.
Objective: To test whether enhanced Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) phosphorylation of RyR2 underlies SR Ca(2+) leak and induction of VT in mdx mice.
Methods: Programmed electrical stimulation was performed on anesthetized mice and confocal imaging of Ca(2+) release events in isolated ventricular myocytes.
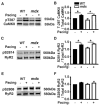
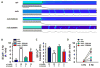
Results: Programmed electrical stimulation revealed inducible VT in mdx mice, which was inhibited by CaMKII inhibition or mutation S2814A in RyR2. Myocytes from mdx mice exhibited more Ca(2+) sparks and Ca(2+) waves compared with wild-type mice, in particular at faster pacing rates. Arrhythmogenic Ca(2+) waves were inhibited by CaMKII but not by protein kinase A inhibition. Moreover, mutation S2814A but not S2808A in RyR2 suppressed spontaneous Ca(2+) waves in myocytes from mdx mice.
Conclusions: CaMKII blockade and genetic inhibition of RyR2-S2814 phosphorylation prevent VT induction in a mouse model of DMD. In ventricular myocytes from mdx mice, spontaneous Ca(2+) sparks and Ca(2+) waves can be suppressed by CaMKII inhibition or mutation S2814A in RyR2. Thus, the inhibition of CaMKII-induced SR Ca(2+) leak might be a new strategy to prevent arrhythmias in patients with DMD without heart failure.
Copyright © 2013 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Groh WJ, Zipes DL. Neurological disorders and cardiovascular disease. In: Bonow RO, Mann DL, Zipes DL, Libby P, editors. Braunwald’s heart disease. A textbook of cardiovascular medicine. Philadephia: Saunders; 2011. pp. 1916–1919.
-
- Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovascular research. 2008;77:766–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

