Imaging and characterization of stretch-induced ATP release from alveolar A549 cells
- PMID: 23247110
- PMCID: PMC3607866
- DOI: 10.1113/jphysiol.2012.244145
Imaging and characterization of stretch-induced ATP release from alveolar A549 cells
Abstract
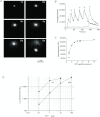
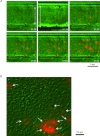
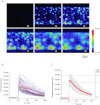
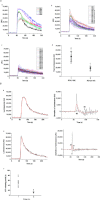
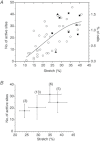
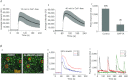
Abstract Mechano-transduction at cellular and tissue levels often involves ATP release and activation of the purinergic signalling cascade. In the lungs, stretch is an important physical stimulus but its impact on ATP release, the underlying release mechanisms and transduction pathways are poorly understood. Here, we investigated the effect of unidirectional stretch on ATP release from human alveolar A549 cells by real-time luciferin-luciferase bioluminescence imaging coupled with simultaneous infrared imaging, to monitor the extent of cell stretch and to identify ATP releasing cells. In subconfluent (<90%) cell cultures, single 1 s stretch (10-40%)-induced transient ATP release from a small fraction (1.5%) of cells that grew in number dose-dependently with increasing extent of stretch. ATP concentration in the proximity (150 μm) of releasing cells often exceeded 10 μm, sufficient for autocrine/paracrine purinoreceptor stimulation of neighbouring cells. ATP release responses were insensitive to the putative ATP channel blockers carbenoxolone and 5-nitro-2-(3-phenylpropyl-amino) benzoic acid, but were inhibited by N-ethylmaleimide and bafilomycin. In confluent cell cultures, the maximal fraction of responding cells dropped to <0.2%, but was enhanced several-fold in the wound/scratch area after it was repopulated by new cells during the healing process. Fluo8 fluorescence experiments revealed two types of stretch-induced intracellular Ca(2+) responses, rapid sustained Ca(2+) elevations in a limited number of cells and delayed secondary responses in neighbouring cells, seen as Ca(2+) waves whose propagation was consistent with extracellular diffusion of released ATP. Our experiments revealed that a single >10% stretch was sufficient to initiate intercellular purinergic signalling in alveolar cells, which may contribute to the regulation of surfactant secretion and wound healing.
Figures




Similar articles
-
Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides.J Physiol. 2007 Oct 15;584(Pt 2):419-35. doi: 10.1113/jphysiol.2007.133314. Epub 2007 Aug 16. J Physiol. 2007. PMID: 17702822 Free PMC article.
-
Type 2 secretory cells are primary source of ATP release in mechanically stretched lung alveolar cells.Am J Physiol Lung Cell Mol Physiol. 2020 Jan 1;318(1):L49-L58. doi: 10.1152/ajplung.00321.2019. Epub 2019 Oct 9. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31596106
-
Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells.Am J Respir Cell Mol Biol. 2014 Dec;51(6):772-82. doi: 10.1165/rcmb.2014-0008OC. Am J Respir Cell Mol Biol. 2014. PMID: 24885163
-
Lamellar body exocytosis by cell stretch or purinergic stimulation: possible physiological roles, messengers and mechanisms.Cell Physiol Biochem. 2010;25(1):1-12. doi: 10.1159/000272046. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054140 Review.
-
ATP release mechanisms, ATP receptors and purinergic signalling along the nephron.Clin Exp Pharmacol Physiol. 2001 Apr;28(4):340-50. doi: 10.1046/j.1440-1681.2001.03451.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11339211 Review.
Cited by
-
Hemolysis is a primary ATP-release mechanism in human erythrocytes.Blood. 2014 Sep 25;124(13):2150-7. doi: 10.1182/blood-2014-05-572024. Epub 2014 Aug 5. Blood. 2014. PMID: 25097178 Free PMC article.
-
Ventilator-induced Lung Injury Promotes Inflammation within the Pleural Cavity.Am J Respir Cell Mol Biol. 2024 Jul;71(1):43-52. doi: 10.1165/rcmb.2023-0332OC. Am J Respir Cell Mol Biol. 2024. PMID: 38767348 Free PMC article.
-
Lung Purinoceptor Activation Triggers Ventilator-Induced Brain Injury.Crit Care Med. 2019 Nov;47(11):e911-e918. doi: 10.1097/CCM.0000000000003977. Crit Care Med. 2019. PMID: 31567350 Free PMC article.
-
ATP release, generation and hydrolysis in exocrine pancreatic duct cells.Purinergic Signal. 2015 Dec;11(4):533-50. doi: 10.1007/s11302-015-9472-5. Epub 2015 Oct 2. Purinergic Signal. 2015. PMID: 26431833 Free PMC article.
-
Alveolar cells under mechanical stressed niche: critical contributors to pulmonary fibrosis.Mol Med. 2020 Oct 14;26(1):95. doi: 10.1186/s10020-020-00223-w. Mol Med. 2020. PMID: 33054759 Free PMC article. Review.
References
-
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. - PubMed
-
- Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975;32:736–745. - PubMed
-
- Bennett MR, Farnell L, Gibson WG. Quantal transmission at purinergic synapses: stochastic interaction between ATP and its receptors. J Theor Biol. 1995;175:397–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

