Age-dependent chloride channel expression in skeletal muscle fibres of normal and HSA(LR) myotonic mice
- PMID: 23247112
- PMCID: PMC3607876
- DOI: 10.1113/jphysiol.2012.246546
Age-dependent chloride channel expression in skeletal muscle fibres of normal and HSA(LR) myotonic mice
Abstract
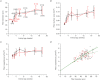
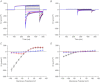
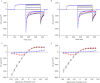
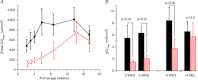
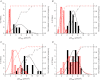
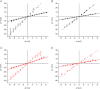
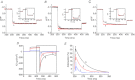
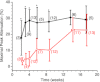
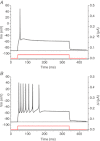
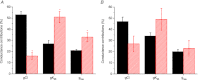
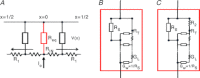
Abstract We combine electrophysiological and optical techniques to investigate the role that the expression of chloride channels (ClC-1) plays on the age-dependent electrical properties of mammalian muscle fibres. To this end, we comparatively evaluate the magnitude and voltage dependence of chloride currents (ICl), as well as the resting resistance, in fibres isolated from control and human skeletal actin (HSA)(LR) mice (a model of myotonic dystrophy) of various ages. In control mice, the maximal peak chloride current ([peak-ICl]max) increases from -583 ± 126 to -956 ± 260 μA cm(-2) (mean ± SD) between 3 and 6 weeks old. Instead, in 3-week-old HSA(LR) mice, ICl are significantly smaller (-153 ± 33 μA cm(-2)) than in control mice, but after a long period of ∼14 weeks they reach statistically comparable values. Thus, the severe ClC-1 channelopathy in young HSA(LR) animals is slowly reversed with aging. Frequency histograms of the maximal chloride conductance (gCl,max) in fibres of young HSA(LR) animals are narrow and centred in low values; alternatively, those from older animals show broad distributions, centred at larger gCl,max values, compatible with mosaic expressions of ClC-1 channels. In fibres of both animal strains, optical data confirm the age-dependent increase in gCl, and additionally suggest that ClC-1 channels are evenly distributed between the sarcolemma and transverse tubular system membranes. Although gCl is significantly depressed in fibres of young HSA(LR) mice, the resting membrane resistance (Rm) at -90 mV is only slightly larger than in control mice due to upregulation of a Rb-sensitive resting conductance (gK,IR). In adult animals, differences in Rm are negligible between fibres of both strains, and the contributions of gCl and gK,IR are less altered in HSA(LR) animals. We surmise that while hyperexcitability in young HSA(LR) mice can be readily explained on the basis of reduced gCl, myotonia in adult HSA(LR) animals may be explained on the basis of a mosaic expression of ClC-1 channels in different fibres and/or on alterations of other conductances.
Figures

Similar articles
-
Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy.J Gen Physiol. 2007 Jan;129(1):79-94. doi: 10.1085/jgp.200609635. Epub 2006 Dec 11. J Gen Physiol. 2007. PMID: 17158949 Free PMC article.
-
Sarcolemmal-restricted localization of functional ClC-1 channels in mouse skeletal muscle.J Gen Physiol. 2010 Dec;136(6):597-613. doi: 10.1085/jgp.201010526. Epub 2010 Nov 15. J Gen Physiol. 2010. PMID: 21078869 Free PMC article.
-
Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy.Mol Cell. 2002 Jul;10(1):35-44. doi: 10.1016/s1097-2765(02)00563-4. Mol Cell. 2002. PMID: 12150905
-
Role of physiological ClC-1 Cl- ion channel regulation for the excitability and function of working skeletal muscle.J Gen Physiol. 2016 Apr;147(4):291-308. doi: 10.1085/jgp.201611582. J Gen Physiol. 2016. PMID: 27022190 Free PMC article. Review.
-
ClC-1 chloride channel: Matching its properties to a role in skeletal muscle.Clin Exp Pharmacol Physiol. 2006 Nov;33(11):1118-23. doi: 10.1111/j.1440-1681.2006.04502.x. Clin Exp Pharmacol Physiol. 2006. PMID: 17042925 Review.
Cited by
-
Use of HSALR female mice as a model for the study of myotonic dystrophy type I.Lab Anim (NY). 2025 Apr;54(4):92-102. doi: 10.1038/s41684-025-01506-7. Epub 2025 Feb 27. Lab Anim (NY). 2025. PMID: 40016516 Free PMC article.
-
Inward rectifier potassium currents in mammalian skeletal muscle fibres.J Physiol. 2015 Mar 1;593(5):1213-38. doi: 10.1113/jphysiol.2014.283648. Epub 2015 Feb 4. J Physiol. 2015. PMID: 25545278 Free PMC article.
-
Muscleblind-Like 1 and Muscleblind-Like 3 Depletion Synergistically Enhances Myotonia by Altering Clc-1 RNA Translation.EBioMedicine. 2015 Jul 31;2(9):1034-47. doi: 10.1016/j.ebiom.2015.07.028. eCollection 2015 Sep. EBioMedicine. 2015. PMID: 26501102 Free PMC article.
-
Transplantation studies reveal internuclear transfer of toxic RNA in engrafted muscles of myotonic dystrophy 1 mice.EBioMedicine. 2019 Sep;47:553-562. doi: 10.1016/j.ebiom.2019.08.031. Epub 2019 Aug 21. EBioMedicine. 2019. PMID: 31446083 Free PMC article.
-
Alternative splicing dysregulation across tissue and therapeutic approaches in a mouse model of myotonic dystrophy type 1.Mol Ther Nucleic Acids. 2024 Sep 13;35(4):102338. doi: 10.1016/j.omtn.2024.102338. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39391766 Free PMC article.
References
-
- Aromataris EC, Rychkov GY. ClC-1 chloride channel: matching its properties to a role in skeletal muscle. Clin Exp Pharmacol Physiol. 2006;33:1118–1123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

