One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines
- PMID: 23247939
- PMCID: PMC3787691
- DOI: 10.7326/0003-4819-157-12-201212180-00005
One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines
Abstract
Background: Guidelines from the United Kingdom and the United States on risk stratification after polypectomy differ, as do recommended surveillance intervals.
Objective: To compare risk for advanced colorectal neoplasia at 1-year colonoscopy among patients cross-classified by U.S. and U.K. surveillance guidelines.
Design: Pooled analysis of 4 prospective studies between 1984 and 1998.
Setting: Academic and private clinics in the United States.
Patients: 3226 postpolypectomy patients with 6- to 18-month follow-up colonoscopy.
Measurements: Rates of advanced neoplasia (an adenoma ≥1 cm, high-grade dysplasia, >25% villous architecture, or invasive cancer) at 1 year, compared across U.S. and U.K. risk categories.
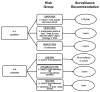
Results: Advanced neoplasia was detected 1 year after polypectomy in 3.8% (95% CI, 2.7% to 4.9%) of lower-risk patients and 11.2% (CI, 9.8% to 12.6%) of higher-risk patients by U.S. criteria. According to U.K. criteria, 4.4% (CI, 3.3% to 5.4%) of low-risk patients, 9.9% (CI, 8.3% to 11.5%) of intermediate-risk patients, and 18.7% (CI, 14.8% to 22.5%) of high-risk patients presented with advanced neoplasia; U.K. high-risk patients comprised 12.1% of all patients. All U.S. lower-risk patients were low-risk by U.K. criteria; however, more patients were classified as low-risk, because the U.K. guidelines do not consider histologic features. Higher-risk U.S. patients were distributed across the 3 U.K. categories. Among all patients with advanced neoplasia, 26.3% were reclassified by the U.K. criteria to a higher-risk category and 7.0% to a lower-risk category, with a net 19.0% benefiting from detection 2 years earlier. Overall, substitution of U.K. for U.S. guidelines resulted in an estimated 0.03 additional colonoscopy every 5 years per patient.
Limitations: Patients were enrolled 15 to 20 years ago, and quality measures for colonoscopy were unavailable. Patients lacking follow-up colonoscopy or with surveillance colonoscopy after 6 to 18 months and those with cancer or insufficient baseline adenoma characteristics were excluded (2076 of 5302).
Conclusion: Application of the U.K. guidelines in the United States could identify a subset of high-risk patients who may warrant a 1-year clearing colonoscopy without substantially increasing rates of colonoscopy.
Primary funding source: European Union Public Health Programme.
Figures
Comment in
-
One-year risk for advanced colorectal neoplasia.Ann Intern Med. 2013 Apr 16;158(8):638-9. doi: 10.7326/0003-4819-158-8-201304160-00018. Ann Intern Med. 2013. PMID: 23588760 No abstract available.
-
One-year risk for advanced colorectal neoplasia.Ann Intern Med. 2013 Apr 16;158(8):639. doi: 10.7326/0003-4819-158-8-201304160-00019. Ann Intern Med. 2013. PMID: 23588762 No abstract available.
-
Optimizing risk stratification in individuals with a personal history of colorectal adenomas.Gastroenterology. 2013 Sep;145(3):682-4. doi: 10.1053/j.gastro.2013.07.028. Epub 2013 Jul 25. Gastroenterology. 2013. PMID: 23891609 No abstract available.
References
-
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. - PMC - PubMed
-
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–78. - PubMed
-
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. - PubMed
-
- Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352(20):2061–8. - PubMed
-
- Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterology. 2010;105:1301–07. - PubMed
Publication types
MeSH terms
Grants and funding
- CA37287/CA/NCI NIH HHS/United States
- CA95060/CA/NCI NIH HHS/United States
- CA104869/CA/NCI NIH HHS/United States
- CA59005/CA/NCI NIH HHS/United States
- P50 CA095060/CA/NCI NIH HHS/United States
- CA-23074/CA/NCI NIH HHS/United States
- U01 CA046927/CA/NCI NIH HHS/United States
- 04/33/01/DH_/Department of Health/United Kingdom
- P30 CA023074/CA/NCI NIH HHS/United States
- R01 CA104869/CA/NCI NIH HHS/United States
- CA-41108/CA/NCI NIH HHS/United States
- CA23108/CA/NCI NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- P01 CA041108/CA/NCI NIH HHS/United States
- R01 CA059005/CA/NCI NIH HHS/United States
- CA26852/CA/NCI NIH HHS/United States