Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma
- PMID: 23248256
- PMCID: PMC4878104
- DOI: 10.1200/JCO.2012.42.1529
Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma
Abstract
Purpose: The primary objective of this study was to determine whether carboplatin, paclitaxel, and sorafenib (CPS) improve overall survival (OS) compared with carboplatin and paclitaxel (CP) in chemotherapy-naive patients with metastatic melanoma.
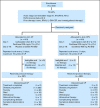
Patients and methods: In this double-blind, randomized, placebo-controlled phase III study, all patients received carboplatin at area under the [concentration-time] curve 6 and paclitaxel 225 mg/m(2) intravenously once every 21 days with random assignment to sorafenib 400 mg orally twice per day on days 2 through 19 every 21 days or placebo. The primary end point was OS, and secondary end points included progression-free survival, objective tumor response, and toxicity.
Results: In all, 823 patients were enrolled over 34 months. At final analysis, the median OS was 11.3 months (95% CI, 9.8 to 12.2 months) for CP and 11.1 months (95% CI, 10.3 to 12.3 months) for CPS; the difference in the OS distribution was not statistically significant by the stratified log-rank test, stratified on American Joint Committee on Cancer (AJCC) stage, Eastern Cooperative Oncology Group (ECOG) performance status, and prior therapy (P = .878). Median progression-free survival was 4.9 months for CPS and 4.2 months for CP (P = .092, stratified log-rank test). Response rate was 20% for CPS and 18% for CP (P = .427). More patients on the CPS arm had grade 3 or higher toxicities (84% v 78%; P = .027), with increased rash, hand-foot syndrome, and thrombocytopenia accounting for most of the difference.
Conclusion: Sorafenib does not improve OS when given in combination with CP for chemotherapy-naive patients with metastatic melanoma. This study establishes benchmark end points for the CP regimen in first-line therapy of metastatic melanoma.
Trial registration: ClinicalTrials.gov NCT00110019.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

