Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities
- PMID: 23248280
- PMCID: PMC3538209
- DOI: 10.1073/pnas.1216516110
Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities
Abstract
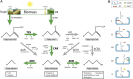
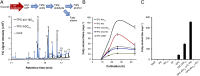
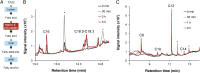
Aliphatic hydrocarbons such as fatty alcohols and petroleum-derived alkanes have numerous applications in the chemical industry. In recent years, the renewable synthesis of aliphatic hydrocarbons has been made possible by engineering microbes to overaccumulate fatty acids. However, to generate end products with the desired physicochemical properties (e.g., fatty aldehydes, alkanes, and alcohols), further conversion of the fatty acid is necessary. A carboxylic acid reductase (CAR) from Mycobacterium marinum was found to convert a wide range of aliphatic fatty acids (C(6)-C(18)) into corresponding aldehydes. Together with the broad-substrate specificity of an aldehyde reductase or an aldehyde decarbonylase, the catalytic conversion of fatty acids to fatty alcohols (C(8)-C(16)) or fatty alkanes (C(7)-C(15)) was reconstituted in vitro. This concept was applied in vivo, in combination with a chain-length-specific thioesterase, to engineer Escherichia coli BL21(DE3) strains that were capable of synthesizing fatty alcohols and alkanes. A fatty alcohol titer exceeding 350 mg·L(-1) was obtained in minimal media supplemented with glucose. Moreover, by combining the CAR-dependent pathway with an exogenous fatty acid-generating lipase, natural oils (coconut oil, palm oil, and algal oil bodies) were enzymatically converted into fatty alcohols across a broad chain-length range (C(8)-C(18)). Together with complementing enzymes, the broad substrate specificity and kinetic characteristics of CAR opens the road for direct and tailored enzyme-catalyzed conversion of lipids into user-ready chemical commodities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
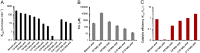

References
-
- Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330(6009):1355–1358. - PubMed
-
- Keasling JD. Synthetic biology and the development of tools for metabolic engineering. Metab Eng. 2012;14(3):189–195. - PubMed
-
- Chan DI, Vogel HJ. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem J. 2010;430(1):1–19. - PubMed
-
- Liu T, Vora H, Khosla C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng. 2010;12(4):378–386. - PubMed
-
- Liu X, Brune D, Vermaas W, Curtiss R., III Production and secretion of fatty acids in genetically engineered cyanobacteria. Proc Natl Acad Sci USA. 2010;108(17):6899–6904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

