Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration
- PMID: 23248282
- PMCID: PMC3538212
- DOI: 10.1073/pnas.1217296110
Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration
Abstract
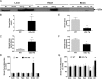
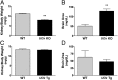
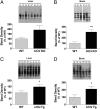
Urate is the end product of purine metabolism in humans, owing to the evolutionary disruption of the gene encoding urate oxidase (UOx). Elevated urate can cause gout and urolithiasis and is associated with cardiovascular and other diseases. However, urate also possesses antioxidant and neuroprotective properties. Recent convergence of epidemiological and clinical data has identified urate as a predictor of both reduced risk and favorable progression of Parkinson's disease (PD). In rodents, functional UOx catalyzes urate oxidation to allantoin. We found that UOx KO mice with a constitutive mutation of the gene have increased concentrations of brain urate. By contrast, UOx transgenic (Tg) mice overexpressing the enzyme have reduced brain urate concentrations. Effects of the complementary UOx manipulations were assessed in a mouse intrastriatal 6-hydroxydopamine (6-OHDA) model of hemiparkinsonism. UOx KO mice exhibit attenuated toxic effects of 6-OHDA on nigral dopaminergic cell counts, striatal dopamine content, and rotational behavior. Conversely, Tg overexpression of UOx exacerbates these morphological, neurochemical, and functional lesions of the dopaminergic nigrostriatal pathway. Together our data support a neuroprotective role of endogenous urate in dopaminergic neurons and strengthen the rationale for developing urate-elevating strategies as potential disease-modifying therapy for PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

