Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family
- PMID: 23248315
- PMCID: PMC3538265
- DOI: 10.1073/pnas.1208863110
Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family
Abstract
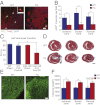
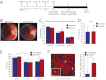
We recently identified a brief time period during postnatal development when the mammalian heart retains significant regenerative potential after amputation of the ventricular apex. However, one major unresolved question is whether the neonatal mouse heart can also regenerate in response to myocardial ischemia, the most common antecedent of heart failure in humans. Here, we induced ischemic myocardial infarction (MI) in 1-d-old mice and found that this results in extensive myocardial necrosis and systolic dysfunction. Remarkably, the neonatal heart mounted a robust regenerative response, through proliferation of preexisting cardiomyocytes, resulting in full functional recovery within 21 d. Moreover, we show that the miR-15 family of microRNAs modulates neonatal heart regeneration through inhibition of postnatal cardiomyocyte proliferation. Finally, we demonstrate that inhibition of the miR-15 family from an early postnatal age until adulthood increases myocyte proliferation in the adult heart and improves left ventricular systolic function after adult MI. We conclude that the neonatal mammalian heart can regenerate after myocardial infarction through proliferation of preexisting cardiomyocytes and that the miR-15 family contributes to postnatal loss of cardiac regenerative capacity.
Conflict of interest statement
Conflict of interest statement: E.N.O. is a cofounder of miRagen Therapeutics, a company focused on developing miRNA-based therapies for cardiovascular disease.
Figures
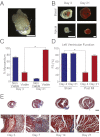
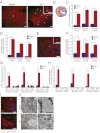
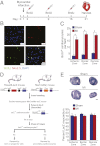
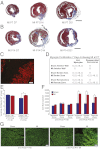
References
-
- Bergmann O, et al. 2009. Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102.
-
- Poss KD, Wilson LG, Keating MT. 2002. Heart regeneration in zebrafish. Science 298(5601):2188–2190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

