Cutaneous adverse drug reaction profile in a tertiary care out patient setting in eastern India
- PMID: 23248414
- PMCID: PMC3523512
- DOI: 10.4103/0253-7613.103304
Cutaneous adverse drug reaction profile in a tertiary care out patient setting in eastern India
Abstract
Background: Cutaneous adverse drug reactions (CADR) are the most frequent of all manifestations of drug sensitivity and manifest with varied and diverse morphology.
Aims: To study the prevalence and clinical spectrum of CADR among patients attending outpatient department (OPD) in a tertiary care hospital.
Materials and methods: An observational study was undertaken over a 1-year period in dermatology OPD of a tertiary care teaching hospital in Eastern India. Patients presenting with suspected drug-related cutaneous lesions were included if drug identity could be ascertained. Clinical profiling was done. Drug history was recorded in a format specified in Indian National Pharmacovigilance Programme and causality assessment carried out as per World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria.
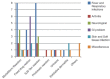
Results: Commonest CADR in our study was morbilliform eruption (30.18%), followed by fixed drug eruption (24.52%), Stevens-Johnson syndrome (SJS)-Toxic epidermal necrolysis (TEN) and overlap of two (24.50%), exfoliative dermatitis (7.54%), urticaria (5.6%), phototoxic drug reaction (3.8%), pityriasis rosea-like eruptions (1.89%), and severe mucositis (1.80%). Drugs implicated were sulfonamides (17%), fixed-dose combinations of fluoroquinolones with nitroimidazoles (11.30%), analgesics (11.30%), antiepileptics (11.30%), beta-lactam antibiotics (9.40%), fluoroquinolones alone (7.50%), allopurinol (7.50%), and azithromycin (5.70%). Reaction latency varied from 1 to 43 days. Causality assessment was certain and probable for 18.9% and 41.5% of the reactions, respectively, and reactions were serious in 33.96% (95% confidence interval 21.21-46.71%).
Conclusions: Cutaneous adverse drug reaction profile in this study is similar in many ways to studies conducted earlier in India. Incidence of life-threatening reactions like SJS-TEN was higher compared with studies conducted abroad. Reaction time and lesion patterns are helpful in identifying an offending drug in the setting of multiple drug therapy.
Keywords: Causality assessment; cutaneous adverse drug reaction; dermatologic pharmacology; pharmacovigilance.
Conflict of interest statement
Figures
References
-
- Sengupta SR, Das NK. Cutaneous adverse drug reaction to systemic drugs: Recent updates. In: Ghosh S, editor. Recent Advances in Dermatology. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; 2004. pp. 88–114.
-
- Uppsala: The Uppsala Monitoring Centre; 2005. [Last accessed 2010 Apr 7]. The use of the WHO-UMC system for standardized case causality assessment [monograph on the Internet] Available from: http://www.who-umc.org/graphics/4409.pdf .
-
- Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol Rev. 2001;53:357–79. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical